Abstract
Research Article
The relationship between serum and sputum levels of azithromycin and clinical endpoints in patients with bronchiectasis using azithromycin maintenance treatment
Josje Altenburg*, Erik B Wilms and Wim G Boersma
Published: 16 July, 2019 | Volume 3 - Issue 1 | Pages: 019-025
Background: Azithromycin (AZM) is a macrolide antibiotic with distinct pharmacokinetic properties and is increasingly used as maintenance treatment in patients with bronchiectasis in order to reduce infectious exacerbations and improve pulmonary symptoms. The exact mechanism of action is not known and the relation between azithromycin dose level, local and systemic drug levels and clinical effect however, has not been extensively studied yet.
Objectives: To explore the relation between AZM serum and sputum concentrations, clinical effect parameters and side effects.
Methods: Azithromycin concentrations were measured in serum and sputum samples of bronchiectasis patients receiving one year of AZM treatment (250mg OD) enrolled in the Bronchiectasis and Azithromycin Treatment (BAT) trial, a double blind, randomised placebo-controlled trial. Results were correlated with data on AZM dose level, exacerbation frequency, lung function (forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), quality of life and symptoms collected within the same year.
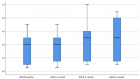
Results: 83 sputum samples from 31 patients and 151 serum samples from 43 patients were available for analysis. Mean AZM dose-level ranged from 18.8 to 39.8 mg/kg body weight/ week, generating mean AZM concentrations of 7.57 mg/L (SD 9.49) in sputum and 0.11 mg/L (SD 0.085) in serum. No correlation was found between side effects and AZM dose level, sputum- or serum concentrations. Significant correlation was found between AZM sputum concentration and CRP-level (r= -0.6).
Conclusion: High and stable AZM sputum levels were reached during long term treatment, as opposed to low AZM levels in serum. Apart from CRP-levels to AZM sputum concentration, no other outcome parameter showed significant correlation to AZM serum- or sputum levels. AZM dose- or exposure levels were not predictive for the occurrence of side effects.
Read Full Article HTML DOI: 10.29328/journal.apps.1001014 Cite this Article Read Full Article PDF
References
- Van BF, Tulkens PM. Macrolides: pharmacokinetics and pharmacodynamics. Int J Antimicrob Agents. 2001; 18: S17-S23. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/11574190
- Di PA, Barbara C, Chella A, Angeletti CA, Del Tacca M. Pharmacokinetics of azithromycin in lung tissue, bronchial washing, and plasma in patients given multiple oral doses of 500 and 1000 mg daily. Pharmacol Res. 2002; 46: 545-550. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/12457629
- Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA Jr, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011; 365: 689-698. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/21864166
- Uzun S, Djamin RS, Kluytmans JA, Mulder PG, van't Veer NE, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014; 2: 361-368. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/24746000
- Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013; 68: 322-329. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/23291349
- Chalmers JD, Elborn JS. Reclaiming the name 'bronchiectasis'. Thorax. 2015; 70: 399-400. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/25791834
- Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. 2013; 309: 1251-1259. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/23532241
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, et al. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993; 16: 5-40. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/8499054
- Altenburg J, Wortel K, de Graaff CS, van der Werf TS, Boersma WG. Validation of a visual analogue score (LRTI-VAS) in non-CF bronchiectasis. Clin Respir J. 2016; 10: 168-175. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/25103370
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD. 2005; 2: 75-79. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/17136966
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992; 145: 1321-1327. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/1595997
- Wilson CB, Jones PW, O'Leary CJ, Cole PJ, Wilson R. Validation of the St. George's Respiratory Questionnaire in bronchiectasis. Am J Respir Crit Care Med. 1997; 156: 536-541. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/9279236
- Bhalla M, Turcios N, Aponte V, Jenkins M, Leitman BS, et al. Cystic fibrosis: scoring system with thin-section CT. Radiology. 1991; 179: 783-788. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/2027992
- Beringer P, Huynh KM, Kriengkauykiat J, Bi L, Hoem N, et al. Absolute bioavailability and intracellular pharmacokinetics of azithromycin in patients with cystic fibrosis. Antimicrob Agents Chemother. 2005; 49: 5013-5017. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/16304166
- Wilms EB, Touw DJ, Heijerman HG. Pharmacokinetics and sputum penetration of azithromycin during once weekly dosing in cystic fibrosis patients. J Cyst Fibros. 2008; 7: 79-84. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/17599845
- Wilms EB, Touw DJ, Heijerman HG. Pharmacokinetics of azithromycin in plasma, blood, polymorphonuclear neutrophils and sputum during long-term therapy in patients with cystic fibrosis. Ther Drug Monit. 2006; 28: 219-225. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/16628134
- Carbon C. Clinical relevance of intracellular and extracellular concentrations of macrolides. Infection. 1995; 23: S10-S14. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/7782109
- Liu P, Allaudeen H, Chandra R, Phillips K, Jungnik A, et al. Comparative pharmacokinetics of azithromycin in serum and white blood cells of healthy subjects receiving a single-dose extended-release regimen versus a 3-day immediate-release regimen. Antimicrob Agents Chemother. 2007; 51: 103-109. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/17060516
- Tamaoki J, Isono K, Sakai N, Kanemura T, Konno K. Erythromycin inhibits Cl secretion across canine tracheal epithelial cells. Eur Respir J. 1992; 5: 234-238. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/1559589
- Tagaya E, Tamaoki J, Kondo M, Nagai A. Effect of a short course of clarithromycin therapy on sputum production in patients with chronic airway hypersecretion. Chest. 2002; 122: 213-218. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/12114361
- McCormack J, Bell S, Senini S, Walmsley K, Patel K, et al. Daily versus weekly azithromycin in cystic fibrosis patients. Eur Respir J. 2007; 30: 487-495. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/17537764
- Brown BA, Griffith DE, Girard W, Levin J, Wallace RJ Jr. Relationship of adverse events to serum drug levels in patients receiving high-dose azithromycin for mycobacterial lung disease. Clin Infect Dis. 1997; 24: 958-964. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/9142801
- Wong C, Jayaram L, Karalus N, Eaton T, Tong C, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. 2012; 380: 660-667. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/22901887
- Wilms EB, Touw DJ, Heijerman HG, van der Ent CK. Azithromycin maintenance therapy in patients with cystic fibrosis: a dose advice based on a review of pharmacokinetics, efficacy, and side effects. Pediatr Pulmonol. 2012; 47: 658-665. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/22684985
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. Standardisation of spirometry. Eur Respir J. 2005; 26: 319-338. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/16055882
- Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA. 2013; 309: 1260-1267. PubMed.: https://www.ncbi.nlm.nih.gov/pubmed/23532242
Figures:

Figure 1
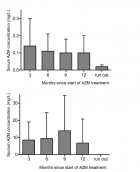
Figure 2

Figure 3
Similar Articles
-
Kinematics and Electromyographic Analysis of Gait with Different FootwearCarlos Alberto Kelencz*,Ingrid Solange Sepúlveda Muñoz,Paulo Rui de Oliveira,Bruno Mazziotti,Cesar Ferreira Amorim. Kinematics and Electromyographic Analysis of Gait with Different Footwear. . 2017 doi: 10.29328/journal.hps.1001001; 1: 001-006
-
A Further Example Showing Efficiency of a Modeling Method Based on the Theory of Dynamic Systems in PharmacokineticsMaria Durisova*. A Further Example Showing Efficiency of a Modeling Method Based on the Theory of Dynamic Systems in Pharmacokinetics. . 2017 doi: 10.29328/journal.hps.1001002; 1: 007-012
-
Sense and antisense Oligodeoxynucleotides to Glun1 Nmdar are Cognitive Enhancers (Nootropics) and protective agents in normal and ischemic (Anoxic) conditions-In vitro studyAnatoly A Mokrushin*. Sense and antisense Oligodeoxynucleotides to Glun1 Nmdar are Cognitive Enhancers (Nootropics) and protective agents in normal and ischemic (Anoxic) conditions-In vitro study. . 2017 doi: 10.29328/journal.hps.1001003; 1: 013-023
-
Paediatric Medicines: Formulation ConsiderationsFátima Roque*. Paediatric Medicines: Formulation Considerations. . 2017 doi: 10.29328/journal.hps.1001004; 1: 024-027
-
Nano-formulations for Ophthalmic TreatmentsMahima John,Rajesh N Gacche*. Nano-formulations for Ophthalmic Treatments. . 2017 doi: 10.29328/journal.hps.1001005; 1: 028-035
-
Diazepam Withdrawal Expression is related to Hippocampal NOS-1 UpregulationEmilce Artur de la Villarmois,María Florencia Constantin,Claudia Bregonzio,Mariela Fernanda Pérez*. Diazepam Withdrawal Expression is related to Hippocampal NOS-1 Upregulation. . 2018 doi: 10.29328/journal.hps.1001006; 2: 001-009
-
Preparation, solid state characterization and evaluation of ketoprofen-glucosamine HCl solid dispersionsAbdul Wahab,Gul Majid Khan*,Mohsen Sharifi,Ahmad Khan,Amjad Khan,Naqab Khan. Preparation, solid state characterization and evaluation of ketoprofen-glucosamine HCl solid dispersions. . 2018 doi: 10.29328/journal.apps.1001007; 2: 010-019
-
Biologic therapy-Related demyelinating peripheral neuropathy in a child with Juvenile Idiopathic ArthritisHeba A Alqurashi,Ghada Al-Salmi,Mohammad A Al-Muhaizea,Sulaiman M Al-Mayouf*. Biologic therapy-Related demyelinating peripheral neuropathy in a child with Juvenile Idiopathic Arthritis. . 2018 doi: 10.29328/journal.apps.1001008; 2: 020-022
-
Some Aspects of medicine distribution in SudanAbdeen Mustafa Omer*. Some Aspects of medicine distribution in Sudan. . 2018 doi: 10.29328/journal.apps.1001009; 2: 023-050
-
Preclinical studies for a cationic liposome formulation containing Il-2 Intended for the treatment of Human TumorsMaria Teresa Corona-Ortega*,Arturo Valle-Mendiola,Leonor Aguilar-Santelises,Araceli Garcia del Valle,Rosalva Rangel-Corona,Benny Weiss-Steider. Preclinical studies for a cationic liposome formulation containing Il-2 Intended for the treatment of Human Tumors. . 2018 doi: 10.29328/journal.apps.1001010; 2: 051-059
Recently Viewed
-
Improvements in the Subjective Sleep of Japanese Middle-aged Managers from the Consumption of an Edible Film Containing CrocetinYusuke Osuka, Hirofumi Masutomi*, Shuji Nakamura, Chiemi Tanigawa, Katsuyuki Ishihara, Masashi Yanagisawa, Toshio Kokubo. Improvements in the Subjective Sleep of Japanese Middle-aged Managers from the Consumption of an Edible Film Containing Crocetin. Arch Food Nutr Sci. 2023: doi: 10.29328/journal.afns.1001054; 7: 088-096
-
Giant Adult-onset Juvenile Xanthogranuloma in an Unusual LocationYassine Tlili*,Aymen Mabrouk,Bassem Mroua,Anis Ben Dhaw,Ghassen Hamdi Kbir,Mounir Ben Moussa. Giant Adult-onset Juvenile Xanthogranuloma in an Unusual Location. Arch Surg Clin Res. 2025: doi: ; 9: 025-026
-
The Rarest Case of Acute Bulbar Palsy due to Internal Jugular Vein Thrombosis Secondary to Protein S Deficiency: Vernet SyndromeRichmond Ronald Gomes*. The Rarest Case of Acute Bulbar Palsy due to Internal Jugular Vein Thrombosis Secondary to Protein S Deficiency: Vernet Syndrome. J Neurosci Neurol Disord. 2025: doi: 10.29328/journal.jnnd.1001110; 9: 052-055
-
Impact of Moringa oleifera Leaf Flour supplement on Weight Gain in Moderately Acutely Malnourished Children in BeninLaleye Flora Tinouade Founmilayo*, Fanou Fogny Nadia and Kayode Polycarpe. Impact of Moringa oleifera Leaf Flour supplement on Weight Gain in Moderately Acutely Malnourished Children in Benin. Arch Food Nutr Sci. 2023: doi: 10.29328/journal.afns.1001052; 7: 070-077
-
Improvement of the Deterioration of the Gut Microbiota Due to High-fat, High-sucrose Diet Acylated Steryl-β-glycosides IntakeMasaki Iji, Kuniyuki Yamada, Yuta Yamane, Chihiro Watanabe, Kazuhito Takemoto, Mamoru Tanaka, Yuichiro Takei, Takako Miyaue, Yoichi Miura, Hiroyuki Watanabe*. Improvement of the Deterioration of the Gut Microbiota Due to High-fat, High-sucrose Diet Acylated Steryl-β-glycosides Intake. Arch Food Nutr Sci. 2023: doi: 10.29328/journal.afns.1001051; 7: 065-069
Most Viewed
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic ReviewMohammad Hossein Karami*, Majid Abdouss*, Mandana Karami. Evaluation of In vitro and Ex vivo Models for Studying the Effectiveness of Vaginal Drug Systems in Controlling Microbe Infections: A Systematic Review. Clin J Obstet Gynecol. 2023 doi: 10.29328/journal.cjog.1001151; 6: 201-215
-
Prospective Coronavirus Liver Effects: Available KnowledgeAvishek Mandal*. Prospective Coronavirus Liver Effects: Available Knowledge. Ann Clin Gastroenterol Hepatol. 2023 doi: 10.29328/journal.acgh.1001039; 7: 001-010
-
Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian RandomizationYong-Qing Zhu, Xiao-Yan Meng, Jing-Hua Yang*. Causal Link between Human Blood Metabolites and Asthma: An Investigation Using Mendelian Randomization. Arch Asthma Allergy Immunol. 2023 doi: 10.29328/journal.aaai.1001032; 7: 012-022
-
An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergiesNathalie Cottel,Aïcha Dieme,Véronique Orcel,Yannick Chantran,Mélisande Bourgoin-Heck,Jocelyne Just. An algorithm to safely manage oral food challenge in an office-based setting for children with multiple food allergies. Arch Asthma Allergy Immunol. 2021 doi: 10.29328/journal.aaai.1001027; 5: 030-037

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."