Abstract
Research Article
Next Generation Tools in mRNA Purification: The Role of Continuous Raman Spectroscopy Testing with Pretreatment of the Sample
Luisetto M*, Nili B Ahmadabadi, Khaled Edbey and Oleg Yurevich Latyshev
Published: 29 March, 2024 | Volume 8 - Issue 1 | Pages: 021-023
In the biopharmaceutical production field, the purification process is a crucial step in order to obtain Drugs with an impurity profile according to the regulatory agency requirement.
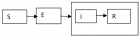
The aim of this work is to verify some relevant and recent literature and after analysis to submit to the researcher new Solutions in order to improve global safety and the toxicological profile: Submit a project related to the continuous testing of the purified materials using Raman spectroscopy – with pre-treatment of the sample: using solvents.
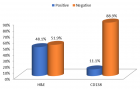
Nanolipis Payload of Biopharmaceutical is not efficiently detected by direct Raman spectroscopy allowed by the regulatory agency for PAT process analytical technology.
Read Full Article HTML DOI: 10.29328/journal.apps.1001052 Cite this Article Read Full Article PDF
Keywords:
mRNA; Purifications; Chromatography; Separations; Columns; Resins; Monoliths; Activated carbon; Raman spectroscopy; Pre-treatment of sample; Continuous testing systems
References
- Campra P. Almeria University https://www.researchgate.net/publication/355979001_DETECTION_OF_GRAPHENE_IN_COVID19_VACCINES-
- Luisetto M, Ahmadabadi NB, Ettarhouni ZO, Edbey K, Latyshev OY. Monoliths in the mRNA Vaccine Purification Process the Silica Resin and Other Composite Materials: The Carbon Content. Int Case Rep Jour. 2022; 2(7):1-23.
- Luisetto M, Edbey K, Tarro G, Ahmadabadi NB, Khan FA. Activated Charcoal and Derivate Materials in Drugs and Biopharmaceutical Purification: Impurity Aspects. J Mater Sci Nanotechnol. 2023; 11(1):105.
- Cipelli RB, Giovannini F, Dark GP. Field Microscopic Analysis on the Blood of 1,006 Symptomatic Persons After Anti-COVID mRNA Injections from Pfizer/BioNtech or Moderna International Journal of Vaccine Theory, Practice, and Research. 2022-08-12. Journal article DOI: 10.56098/ijvtpr.v2i2.47 CONTRIBUTORS:
- Luisetto M. Raman (Rs) Spectroscopy for Biopharmaceutical Quality Control and PAT. Raw Material - Final Products: the Nanolipids Effect on Signal Intensity. Regulatory and Toxicological Aspects. Med & Analy Chem Int J. 2022; 6(1): 000175.
- Vanden-Hehir S, Tipping WJ, Lee M, Brunton VG, Williams A, Hulme AN. Raman Imaging of Nanocarriers for Drug Delivery. Nanomaterials (Basel). 2019 Mar 3;9(3):341. doi: 10.3390/nano9030341. PMID: 30832394; PMCID: PMC6474004.
- Activated charcoal BP monography.
- Luisetto WM. Amorphous substance (activated charcoal) and other impurities in API manufacturing process. Pharmaceutical, toxicological and regulatory implication. 2023.
- Robert OY. Scanning & Transmission Electron Microscopy Reveals Graphene Oxide in CoV-19 Vaccines 2021. February 5th, Updated September 11th, 2021. www.drrobertyoung.com
Figures:
Similar Articles
-
Metabolic profiling and antibacterial activity of Eryngium pristis Cham. & Schltdl. - prospecting for its use in the treatment of bacterial infectionsLaura Silva Fernandes,Ygor Ferreira Garcia da Costa,Martha Eunice de Bessa,Adriana Lucia Pires Ferreira,José Otávio do Amaral Corrêa,Glauciemar Del-Vechio Vieira,Orlando Vieira de Sousa,Ana Lúcia Santos de Matos Araújo,Paula C Castilho*,Maria Silvana Alves*. Metabolic profiling and antibacterial activity of Eryngium pristis Cham. & Schltdl. - prospecting for its use in the treatment of bacterial infections. . 2021 doi: 10.29328/journal.apps.1001027; 5: 020-028
-
Design and optimization of mRNAs encoding an Anti-TIGIT antibody with therapeutic potential for cancer in TIGIT-humanized BALB/c MiceJingmin Cui, Gulisaina Qiaerxie, Hui Qin, Feng Long, Xi Wang, Zhixin Yang, Peng Du*, Yong Cui*. Design and optimization of mRNAs encoding an Anti-TIGIT antibody with therapeutic potential for cancer in TIGIT-humanized BALB/c Mice. . 2023 doi: 10.29328/journal.apps.1001038; 7: 008-016
-
Next Generation Tools in mRNA Purification: The Role of Continuous Raman Spectroscopy Testing with Pretreatment of the SampleLuisetto M*, Nili B Ahmadabadi, Khaled Edbey, Oleg Yurevich Latyshev. Next Generation Tools in mRNA Purification: The Role of Continuous Raman Spectroscopy Testing with Pretreatment of the Sample. . 2024 doi: 10.29328/journal.apps.1001052; 8: 021-023
Recently Viewed
-
Comparison of Effect of Intrathecal Fentanyl 25µg with 0.5% Hyperbaric Bupivacaine and Only 0.5% Hyperbaric BupivacaineParveen Gafla*. Comparison of Effect of Intrathecal Fentanyl 25µg with 0.5% Hyperbaric Bupivacaine and Only 0.5% Hyperbaric Bupivacaine. Int J Clin Anesth Res. 2025: doi: 10.29328/journal.ijcar.1001029; 9: 017-022
-
Impact of Moringa oleifera Leaf Flour supplement on Weight Gain in Moderately Acutely Malnourished Children in BeninLaleye Flora Tinouade Founmilayo*, Fanou Fogny Nadia and Kayode Polycarpe. Impact of Moringa oleifera Leaf Flour supplement on Weight Gain in Moderately Acutely Malnourished Children in Benin. Arch Food Nutr Sci. 2023: doi: 10.29328/journal.afns.1001052; 7: 070-077
-
Drug Rehabilitation Centre-based Survey on Drug Dependence in District Shimla Himachal PradeshKanishka Saini,Palak Sharma,Bhawna Sharma*,Atul Kumar Dubey,Muskan Bhatnoo,Prajkta Thakur,Vanshika Chandel,Ritika Sinha. Drug Rehabilitation Centre-based Survey on Drug Dependence in District Shimla Himachal Pradesh. J Addict Ther Res. 2025: doi: 10.29328/journal.jatr.1001032; 9: 001-006
-
Feature Processing Methods: Recent Advances and Future TrendsShiying Bai,Lufeng Bai*. Feature Processing Methods: Recent Advances and Future Trends. J Clin Med Exp Images. 2025: doi: 10.29328/journal.jcmei.1001035; 9: 010-014
-
Relationship between Vitamin D Deficiency and Lipopolysaccharides Porphyromonas gingivalis Bacteria in Stunting ChildrenErwin Gunawan*,Ria Puspitawati. Relationship between Vitamin D Deficiency and Lipopolysaccharides Porphyromonas gingivalis Bacteria in Stunting Children. Ann Biomed Sci Eng. 2024: doi: 10.29328/journal.abse.1001033; 8: 059-065
Most Viewed
-
Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth EnhancersH Pérez-Aguilar*, M Lacruz-Asaro, F Arán-Ais. Evaluation of Biostimulants Based on Recovered Protein Hydrolysates from Animal By-products as Plant Growth Enhancers. J Plant Sci Phytopathol. 2023 doi: 10.29328/journal.jpsp.1001104; 7: 042-047
-
Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case PresentationJulian A Purrinos*, Ramzi Younis. Sinonasal Myxoma Extending into the Orbit in a 4-Year Old: A Case Presentation. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001099; 8: 075-077
-
Feasibility study of magnetic sensing for detecting single-neuron action potentialsDenis Tonini,Kai Wu,Renata Saha,Jian-Ping Wang*. Feasibility study of magnetic sensing for detecting single-neuron action potentials. Ann Biomed Sci Eng. 2022 doi: 10.29328/journal.abse.1001018; 6: 019-029
-
Pediatric Dysgerminoma: Unveiling a Rare Ovarian TumorFaten Limaiem*, Khalil Saffar, Ahmed Halouani. Pediatric Dysgerminoma: Unveiling a Rare Ovarian Tumor. Arch Case Rep. 2024 doi: 10.29328/journal.acr.1001087; 8: 010-013
-
Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative reviewKhashayar Maroufi*. Physical activity can change the physiological and psychological circumstances during COVID-19 pandemic: A narrative review. J Sports Med Ther. 2021 doi: 10.29328/journal.jsmt.1001051; 6: 001-007

HSPI: We're glad you're here. Please click "create a new Query" if you are a new visitor to our website and need further information from us.
If you are already a member of our network and need to keep track of any developments regarding a question you have already submitted, click "take me to my Query."