Research Article
Preparation, solid state characterization and evaluation of ketoprofen-glucosamine HCl solid dispersions

Abdul Wahab1, Gul Majid Khan2*, Mohsen Sharifi3, Ahmad Khan2, Amjad Khan2 and Naqab Khan4
1Department of Pharmacy, Kohat University of Science and Technology. Kohat 26000, KPK, Pakistan2Department of Pharmacy, Quaid-i-Azam University, Islamabad 45320, Pakistan
31522 Louisiana St., Little Rock, Arkansas 72202, USA
4Department of Biotechnology, Gomal University, Dera Ismail Khan, KPK, Pakistan
*Address for Correspondence: Dr. Gul Majid Khan, Professor, Department of Pharmacy, Quaid-i-Azam University, Islamabad 45320, Pakistan, Email: [email protected]
Dates: Submitted: 13 June 2018; Approved: 25 June 2018; Published: 26 June 2018
How to cite this article: Wahab A, Khan GM, Sharifi M, Khan A, Khan A, et al. Preparation, solid state characterization and evaluation of ketoprofen-glucosamine HCl solid dispersions. Arch Pharm Pharma Sci. 2018; 2: 010-019. DOI: 10.29328/journal.apps.1001007
Copyright License: © 2018 Wahab A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ketoprofen; Glucosamine HCl; Solid dispersions; Physical Mixtures; Solubility; Drug release rate
Abstract
In this investigation, solid dispersions were prepared and characterized to improve the solubility and dissolution of poorly water soluble drug Ketoprofen, using glucosamine HCl as a carrier. For the improvement of the solubility and dissolution rate of poorly water soluble drugs different techniques are used such as solubilization, salt formation, particle size reduction and solid dispersion etc, but in the present study, solid dispersions (SDs) of poorly water soluble NSAID Ketoprofen were prepared to improve its solubility and dissolution rate, using solvent evaporation method with drug-carrier ratio of 1:1, 1:2 and 1:3. Our results indicate that all solid dispersions of Ketoprofen and Glucosamine HCl exhibited more enhancements in solubility and dissolution rates than corresponding physical mixtures. The DSC thermograms and X-ray diffraction patterns showed a slight reduction in crystallinity in solid dispersions which were further verified by FT-IR and SEM. It is concluded that solid dispersion is an effective technique for enhancing the solubility and dissolution rate of poorly water-soluble drug Ketoprofen using Glucosamine HCl as a carrier. This amino sugar (Glucosamine HCl) could be used as a novel potential carrier for preparation and formulation of SDs and would have potential commercial benefits.
Introduction
Due to good patient compliance, convenience and low medicine and production cost, the oral route of drug administration is the most preferred route. For systemic absorption of drug after oral administration it is must for drug to be dissolved in gastrointestinal tract (GIT) fluids [1]. As dissolution is the rate limiting step for the onset of therapeutic and pharmacological effect of drug, therefore, oral bioavailability of a drug depends on dissolution rate and solubility [2]. Thus, during developmental phases of new product to be launched in the market, poor solubility is one of the challenges. According to one estimate 40% of all newly developed drugs or products are either poorly water-soluble or insoluble in water [3]. Therefore, efforts are needed for enhancing the solubility and dissolution rates of poorly water-soluble drugs in order to improve bioavailability and maximize its therapeutic effects.
Different techniques are used, such as solubilization, salt formation, particle size reduction and solid dispersion etc, for improving the dissolution rate and enhancing the solubility of poorly water-soluble drugs [4,5]. Among all of these techniques, SD of drug in water-soluble carrier is one of the promising techniques and has often proved to be the most successful for enhancing the solubility and improving the dissolution rate of poorly water-soluble drugs, because it is simple, easy to handle and economical [1,6]. Solid dispersions are defined as the dispersions of one or more active ingredients in inert carriers at solid state prepared by fusion, solvent or solvent fusion method [7]. Various mechanisms and contributing factors are involved to enhance and increase the solubility and improve dissolution rates of poorly water-soluble drugs, using solid dispersions: eutectic formation, formation of true solid solution, reduction of particle size to submicron or molecular size [1]. Dissolution rate is increased by reduction in particle size. Moreover, in some cases active pharmaceutical ingredients are converted from crystalline forms to amorphous forms which have higher energetic states with enhanced solubility. The dissolved hydrophilic carrier can improve the wettability of some drugs particles [8,9].
Ketoprofen was selected in this study, as a model hydrophobic drug. Ketoprofen [2-(3-benzoylphenyl) propionic acid] is NSAID, widely used for reducing inflammation and pain caused by rheumatoid arthritis, osteoarthritis, spondylitis or abdominal cramp associated with menstruation. It is classified as class II drug of Biopharmaceutical Classification System. It has low water solubility and hence, poor dissolution rate, so to enhance its solubility and dissolution rate SDs of Ketoprofen were formulated and developed, by solvent evaporation technique, using novel and potential carrier Glucosamine HCl. Glucosamine HCl was chosen as a carrier, because it is non-toxic, highly hydrophilic and moreover, it has been used for reduction in pain and improving the mobility in osteoarthritis [10]. As Glucosamine is unstable, therefore, mostly its salts either hydrochloride or sulfate are used in therapy. Our research group has been extensively investigating, for more than a decade, various types of drug delivery systems containing different model drugs, including but not limited to Ketoprofen [11,12]. In the present study we have explored the use of D-Glucosamine HCl as a hydrophilic carrier to enhance the solubility and dissolution rate of hydrophobic drug Ketoprofen Graph 1. Glucosamine HCl was used in this study because it is more stable than Glucosamine Sulfate [10].
Material and Methods
Material
Ketoprofen was purchased from Gratis drug testing laboratory, Pakistan. Glucosamine HCl (Sigma, UK), ethanol (Fisher Scientific, UK), KH2PO4 (Sigma, UK), NaOH (Sigma, UK) were used. All the other reagents and solvents were of analytical grade and were used without further purification.
Preparation of Ketoprofen SDs
SDs of Ketoprofen were prepared with drug and carrier (Glucosamine HCl) 1:1, 1:2 and 1:3 by weight, using solvent evaporation technique [13,14]. The drug was dissolved in ethanol followed by carrier dispersion (Glucosamine) 50 ml in ethanol. The solvent was then removed by evaporation at 40oC by stirring at 100rpm for 24 hours. The solid dispersions prepared were then collected and kept at room temperature for 48 hours. Then the mass was pulverized in porcelain mortar and pestle and passed through sieve no. 100, and stored at room temperature in a desiccator until further use.
Preparation of Ketoprofen physical mixtures
For comparative studies of Ketoprofen solid dispersions, physical mixtures (PMs) were also prepared. The physical mixtures prepared were having the same composition of the solid dispersions; however, they were prepared by simple trituration of drugs and carrier in porcelain mortar followed by thorough blending in poly bags. The mixtures were then sieved and stored in desiccator at room temperature until further evaluation. The composition of physical mixtures and solid dispersions of the model drugs is shown in table 1.
| Table 1: Composition of SDs and PMs of Ketoprofen (KTP). | |||
| Formulation Code | Carrier | Drug : Carrier | Method |
| F1KTP | Glucosamine HCl | 1:1 | Physical mixture (trituration) |
| F2 KTP | Glucosamine HCl | 1:2 | Physical mixture (trituration) |
| F3 KTP | Glucosamine HCl | 1:3 | Physical mixture (trituration) |
| F4 KTP | Glucosamine HCl | 1:1 | Solid dispersion (solvent evaporation) |
| F5 KTP | Glucosamine HCl | 1:2 | Solid dispersion (solvent evaporation) |
| F6 KTP | Glucosamine HCl | 1:3 | Solid dispersion (solvent evaporation) |
Evaluation of SDs and PMs of Ketoprofen
The evaluation of SDs and PMs was performed using the following different techniques:
Drug Content Determination
The drug content in each formulation was determined by taking the SDs or PMs equivalent to 50mg of the respective model drug (Ketoprofen) and transferring it to 100 ml volumetric flask and then small volume (10ml) of phosphate buffer (pH 7.4) was added to hydrate the samples. Finally the volume was made up to the mark. The samples were shaken for some time to dissolve the drug completely and were filtered carefully. The absorbance values of standard (Ketoprofen, supplied by Sanofi Aventis, Islamabad, Pakistan) and the samples were determined at UV spectrum (λmax) 258 nm, using double beam spectrophotometer (UV-1601, Shimadzu, Japan). Three reading were taken and then mean and standard deviation were calculated.
Differential scanning calorimetry (DSC) studies
The DSC study of carrier Glucosamine, pure Ketoprofen, the SDs and physical mixtures of the model drug was performed using DSC instrument (Mettler Toledo DSC 822e) equipped with Star© computer program. Approximately 3-6mg of sample was weighed in aluminum pan and then sealed with punched lid. The temperature ranged between 20-300oC with heating rate of 10oC/min under nitrogen gas flow.
Fourier transform Infrared (FT-IR) studies
The FT-IR spectra of carrier Glucosamine, pure Ketoprofen, the solid dispersions and physical mixtures were taken to observe the drug-carrier interaction, using FT-IR Spectrum One spectrophotometer (Perkin Elimer, UK) in the range of 650 to 4000 cm-1. The sample of several milligrams was placed on the stage of machine and then handle of the machine was placed on the sample for generation of enough pressure. Sharp peaks with reasonable intensities were obtained. The spectra obtained were the result of 4 scans at 1 cm-1 resolution.
X-ray powder diffractometry studies
X-ray patterns of pure Ketoprofen, physical mixtures and solid dispersions were taken using a Philips PW 1830 powder diffractometer (Philips, Eindhoven, Netherlands). The prepared samples were exposed to Cu Kα radiation (λ=1.5418 Å) in the range of 00 ≤ 2θ ≤ 500. The step size was 0.050 and the time for each step was kept two seconds.
Scanning electron microscope (SEM) analysis
Electron micrographs of carrier Glucosamine, pure Ketoprofen, PMs and SDs were obtained using SEM (SEM; Joel JSM-5910, Japan) operating at 10 kV. The samples prepared were mounted on a metal’s stub using adhesive tape with double sided and gold was used as coating material for conductivity in an organ atmosphere before observation. To study the morphology of active drugs, PMs and SDs, micrographs with different magnification were obtained.
Solubility measurement
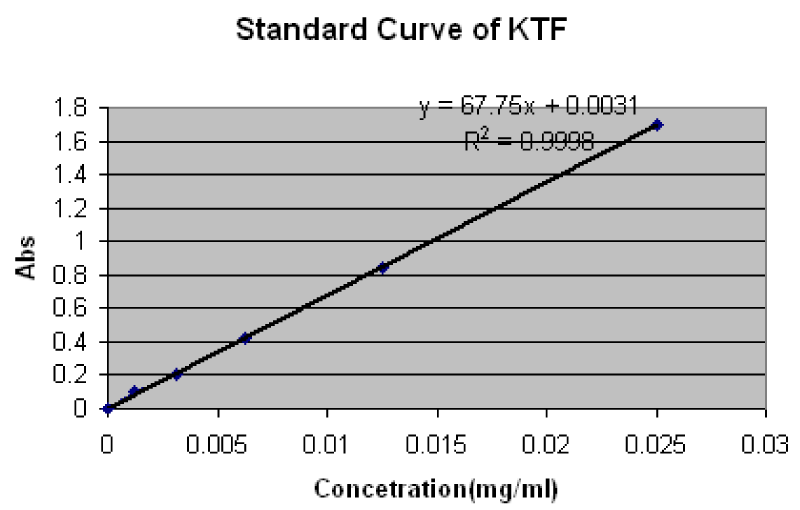
The solubility measurements of pure Ketoprofen, PMs and SDs in distilled water were performed according to the well published method by Higuchi and Connors (1965). Accordingly, surplus amount (100mg) of Ketoprofen, PMs and SDs were placed in 100ml volumetric flasks and then final volume was made-up to 100 ml with distilled water. The flasks were sealed with aluminum foils using rubber bands to avoid solvent loss. Then these flasks were kept on shaking using thermostatically controlled shaking water bath (Shel Lab, 1217-2E, USA) for 24 hours at room temperature (25oC). The oscillation speed was kept at 100 oscillations per minute. After 24 hours all flasks were kept undisturbed on flat surface for three hours. A few ml supernatant from each flask was taken and filtered through membrane filter (0.45µm). One mL of each filtrate was diluted with the same distilled water up to 25ml. The diluted samples were evaluated to determine the Ketoprofen solubility, using a UV/Visible double beam spectrophotometer (Shmadzu, 1601, Japan) at λmax 258 nm. The calibration curve (Standard Curve) was used for the determination of the quantity of soluble drug per ml (Supplementary Material 1).
In vitro dissolution studies
The in-vitro dissolution studies were conducted by USP method II (Paddle method) using eight stations dissolution apparatus Pharma Test (PTWS-11/P, TPT, Hunburg, Germany) and the rotation speed of paddles was set at 100 r. p.m. Each station or flask of the dissolution apparatus was filled with 900ml of distilled water used as dissolution medium to study percentage dissolution of model drugs (Ketoprofen), PMs and SDs. The temperature of dissolution medium was kept 37oC ± 0.5oC. Samples of five ml at selected time intervals, such as 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90, 100, 110, 120 min were withdrawn with the help of syringes consisting of 0.45µm filters. After each sampling equal volume of fresh dissolution medium was substituted to retain the dissolution medium constant. Then after appropriate dilution the samples were analyzed for Ketoprofen, using double beam spectrophotometer (UV-1601, Shimadzu, Japan) at λmax 258nm. Percent drug dissolution of Ketoprofen was calculated by using calibration standard curves of the drug. The study was conducted in triplicate.
Results
Preparation of solid dispersions
Different methods, such as salt formation, solubilization, particle size reduction, complex formation, solvent evaporation, etc. are used to prepare SDs to enhance the dissolution rate and thereby, improve the bioavailability of poorly water-soluble drugs [4], however, in this study solvent evaporation method was used due to its inherent ease of handling and no more steps were required. The SDs of Ketoprofen with different drug-carrier ratios was prepared. The respective PMs with the same drug and carrier ratios were prepared by simple trituration technique for comparative evaluation.
For confirmation of uniform dispersion of drug in solid dispersions and physical mixtures drug content analysis was performed and it was found between 99.57±0.7 % and 101.3±0.32 % respectively. Similar studies were conducted by [13,15], who prepared solid dispersions of Tebinanfine hydrochloride and NSAIDs by the same method obtaining good results in terms of content analysis and uniform distribution of the drugs used.
Solubility study
As shown in table 2, the aqueous solubility study of pure Ketoprofen, their PMs and SDs was performed in distilled water. The study showed that solubility of Ketoprofen was enhanced in presence of carrier (Glucosamine HCL). This effect of solubility enhancement was more prominent in case of SDs as compared to that of their respective PMs (Table 2). The enhancement of drugs solubility in presence of solid dispersions may be due to conversion of drugs to amorphous form as amorphic forms of drug are more soluble than their crystalline form [13,16]. The increase in solubility of drugs in SDs might also be due to good wettability and dispersability [16].
| Table 2: Solubility data of different Ketoprofen formulations. | |
| Formulations | Solubility(mg/ml) |
| KPT Pure | 0.221 |
| F1 KPT | 0.335 |
| F2 KPT | 0.355 |
| F3 KPT | 0.377 |
| F4 KPT | 0.465 |
| F5 KPT | 0.501 |
| F6 KPT | 0.555 |
Differential scanning calorimetry (DSC) studies
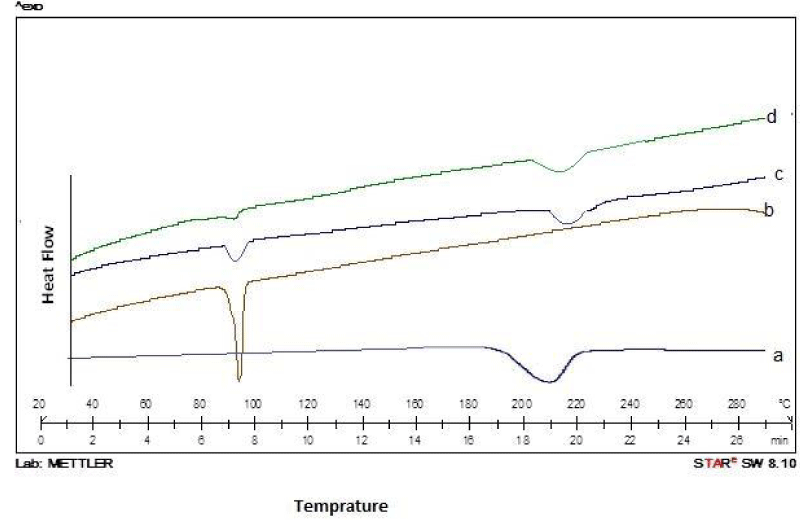
Differential scanning calorimetry (DSC) studies of pure ketoprofen, their physical mixtures and solid dispersions were conducted to investigate the drug- carrier interaction. As shown in (Figure 1a-d), the DSC studies of pure Ketoprofen, its PMs and SDs exhibited interactive effects. The pure Ketoprofen and carrier (Glucosamine HCL) showed sharp endothermic peaks round 94oC and 210oC, corresponding to their melting points, while the DSC thermograms of Ketoprofen in physical mixtures (MPs) and solid dispersion (SDs) (Figure 1c-d) did not exhibit any major change in the endothermic peaks except disappearance of sharpness, indicating occurrence of no possible interaction between Ketoprofen and Glucosamine HCl.
Figure 1: DSC Thermograms of (a) Glucosamine; (b) Pure ketoprofen; (c) Physical mixture; and (d) Solid dispersions of ketoprofen with glucosamine.
Our results are supported by findings in other studies previously conducted by several investigators [16,17].
Fourier transform Infrared (FT-IR) studies
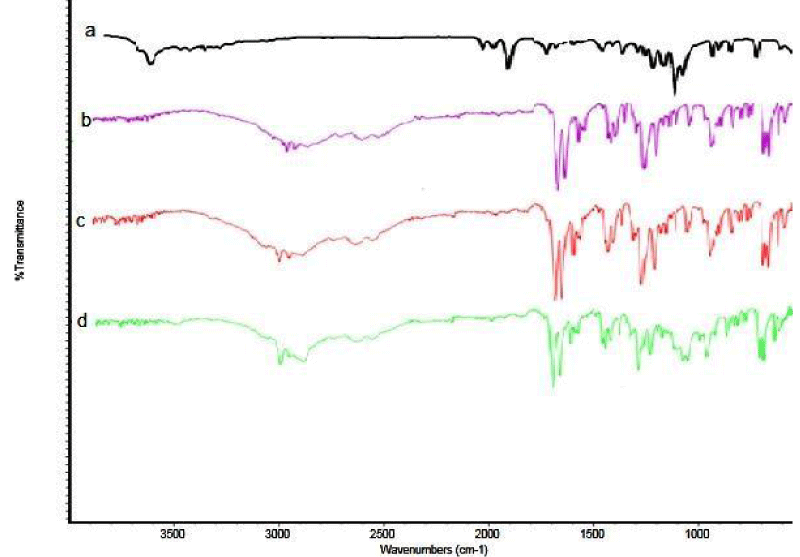
For the confirmation of interaction between drugs and carrier in presence of PMs and SDs, FT-IR studies were performed. Pure ketoprofen crystals show two carbonyl absorption bands at 1694.4 cm-1 and 1654.2 cm-1, indicating carboxyl carbonyl and ketonic carbonyl stretching, respectively [18,19], as shown in Figure 2a-d. The characteristics stretching band of pure ketoprofen with Glucosamine in PM and SDs did not change, and on the basis of these observations no possible interaction was observed between Ketoprofen and Glucosamine HCl. The same findings were observed by other researchers [20].
Figure 2: DSC Thermograms of (a) Glucosamine; (b) Pure ketoprofen; (c) Physical mixture; and (d) Solid dispersions of ketoprofen with glucosamine.
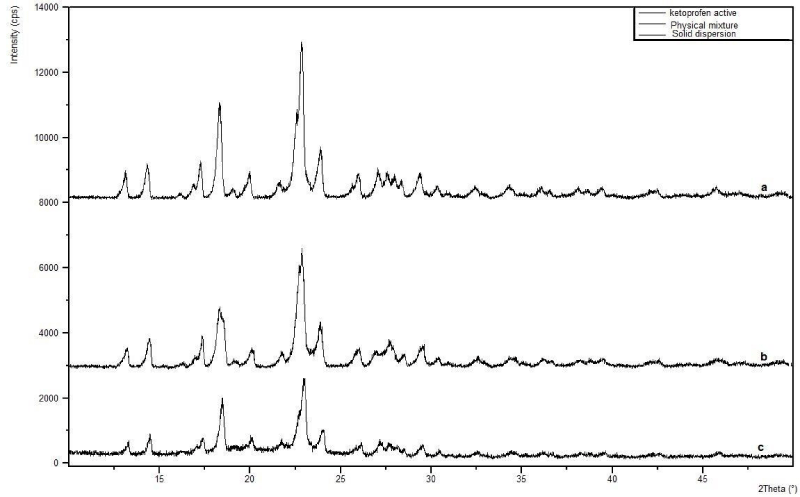
X-ray diffractometry studies
As shown in Figure (3a-c) different peaks with high intensity were present in the diffractogram around 13o, 14o, 18o, 23o, 24o, 26o and 29o along with some other peaks of lower intensity. The same peaks were present in the diffractograms PM and SD but with low intensities, indicating the conversion of crystalline form of pure Ketoprofen to amorphous form in presence of PM and SDs. Moreover, the peak intensity of Ketoprofen was much more reduced as compared to pure Ketoprofen and PM, as shown in Figure 3b-c. Our results confirm the findings of other researchers [17-20].
Figure 3: X-ray diffractograms of (a) Pure ketoprofen; (b) Physical mixture; and (c) Solid dispersions of ketoprofen and glucosamine.
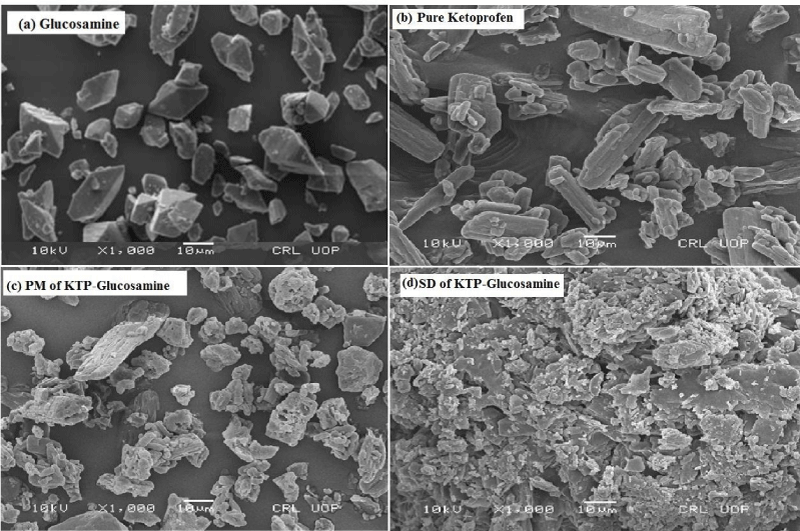
Scanning electron microscope analysis
Presented in Figure 4a-d, Glucosamine and pure Ketoprofen has prismatic shape (polygonal) and irregular shape crystals, respectively. The SEM analysis showed that relatively smaller polyhedral crystalline forms of Glucosamine and Ketoprofen are clearly visible in PM, as shown in Figure 4c, the Ketoprofen has smallest, irregular, circular and plate like crystals in SDs, which are responsible for enhanced dissolution rate. Similar studies were conducted [17,20]. Our results confirm their findings.
Figure 4: Scanning electron photomicrographs of (a) Carrier (Glucosamine HCl); (b) Pure Ketoprofen; (c) Physical mixture of Ketoprofen-Glucosamine HCl; (d) Solid dispersion of Ketoprofen-Glucosamine HCl.
In vitro dissolution studies
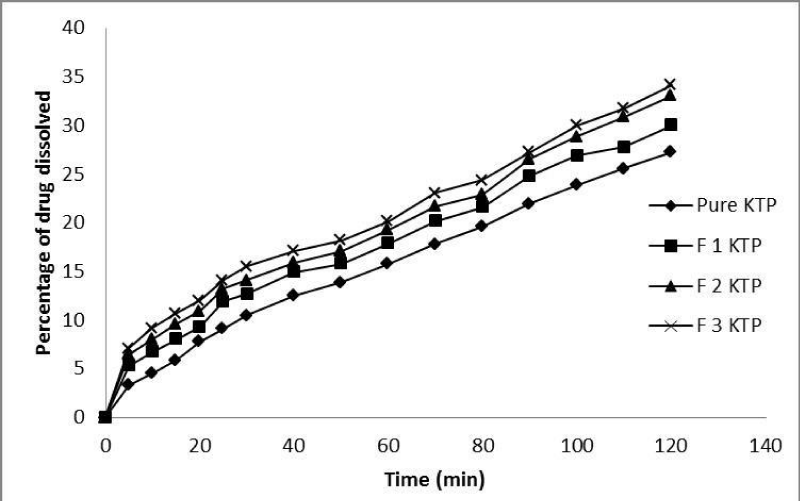
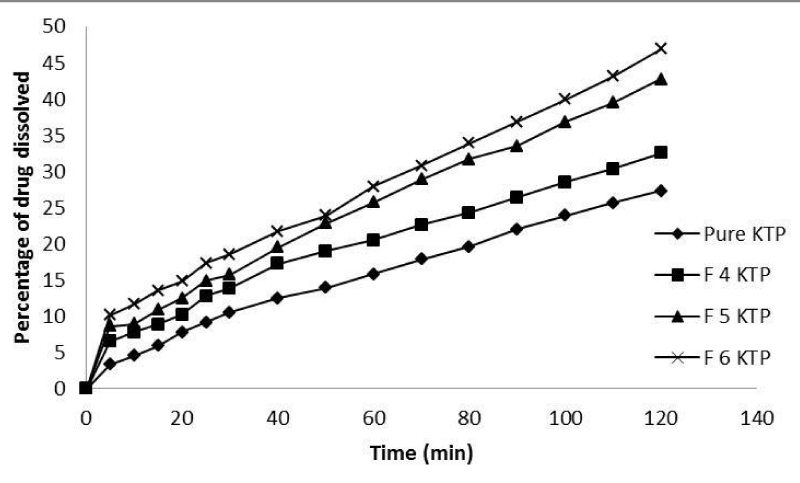
The in-vitro dissolution study was performed for Ketoprofen, Ketoprofen PMs and SDs. The dissolution profile of pure Ketoprofen, Ketoprofen PM and SDs with Glucosamine HCl are depicted in Figures 5,6 respectively. As shown 27.3±0.324% of pure Ketoprofen was dissolved after 120 minutes, while in case of physical mixtures and solid dispersions with different drug: carrier ratios (1:1, 1:2 and 1:3) the dissolution rate was linearly increased and 30±0.932%, 33.1±0.543% and 34.2±0.321% and 32.5±0.321%, 42.7±0.654% and 46.9±0.765% of drug was dissolved after 120 minutes from formulations F1 KTP, F2 KTP, F3 KTP and F4 KTP, F5 KTP, F6 KTP, respectively. The fast and rapid dissolution rate of Ketoprofen in SDs may be due to the presence of Ketoprofen in amorphous form which is revealed by the results of different techniques as mentioned above. On the other hand it may be, that if the percentage (quantity) of carrier is too high, this may cause increase in solubility and dissolution rate due to absence of crystallinity of drug [21].
Figure 5: In vitro dissolution profiles of pure Ketoprofen and physical mixture with different drug-carrier (Glucosamine HCl) ratio.
Figure 6: In vitro dissolution profiles of pure Ketoprofen and solid dispersion with different drug-carrier (Glucosamine HCl) ratio.
Discussion
The SDs were prepared of Ketoprofen, using Glucosamine HCl as hydrophilic carrier by solvent evaporation method with drug carrier ratio 1:1, 1:2, and 1:3. The results depicted that 1:3 (F6 KTP) solid dispersions have the highest dissolution profile as compared to pure Ketoprofen and other formulations. The order of dissolution rates of pure Ketoprofen and different formulations is, pure Ketoprofen ˂ F1 KPT ˂ F2 KPT ˂ F3 KPT ˂ F4 KPT ˂ F5 KPT ˂ F6 KPT. The enhancement of the dissolution rate of solid dispersions can be ascribed to various factors such as lack of crystallinity (amorphization), particle size reduction, increased wettability and dispersability [21,22]. As shown by dissolution data of the PMs, improvement could be attributed higher dispersability and wettability. Therefore, PMs of drug with hydrophilic carrier result in greater wetting as compared to pure Ketoprofen. Different techniques were used for solid state characterizations and determination of interactions between drug and carrier. Differential scanning caloromitry studies, on the basis of melting point of Ketoprofen and carrier confirmed that there is no possible interaction between drug and carrier. However, broadness of characteristic peaks with reduced peak intensity for both PMs and SDs may be attributed higher carrier concentration and uniform distribution of the drug in carrier, resulting in complete miscibility of the drug carrier. Similar study was conducted by other researchers Chaulang and colleagues [2], using sodium starch glycolate for the preparation of Furosemide SDs. The DSC finding were also confirmed by FT-IR, XRD and SEM studies, as shown in FT-IR spectroscopic studies, the spectra of pure Ketoprofen, PM and SDs is similar and the peaks of Ketoprofen appeared in mixture and solid dispersion. Thus, there was no possible interaction found between drug and carrier. Furthermore, the above findings were also confirmed by XRD studies, the diffractogram of pure Ketoprofen exhibited series of intense peaks which are indicative of their crystallinity, while PM and SD prepared showed reduction in sharpness of the XRD peaks intensity, indicating the conversion of drug from crystalline from to amorphous form. For further confirmation SEM studies were conducted, prismatic shape (polygonal) and irregular shape crystals were found for Ketoprofen and Glucosamine HCl, respectively. The SEM analysis showed that relatively smaller polyhedral crystalline forms of Glucosamine and Ketoprofen are clearly visible in physical mixture, while irregular, circular and plate like crystals were shown in solid dispersions, which are responsible for enhanced dissolution rates.
Conclusion
The study presented shows that the dissolution rate and solubility of poorly water-soluble drug Ketoprofen can be increased considerably, using Glucosamine Hydrochloride as a hydrophilic carrier by formulating it as a solid dispersion. It was shown that Glucosamine-HCl exhibited a prominent role in the enhancement of dissolution rate and solubility of Ketoprofen. The present study depicted that Glucosamine-HCl could be used as a novel carrier in SD formulations with potential commercial out comes. For solid state characterization of pure drug, solid dispersion and physical mixture different techniques were used such as differential scanning calorimeter (DSC), Fourier transform infrared spectroscopy (FT-IR), x-ray diffractometry (XRD) and scanning electron microscopy (SEM), and all formulations developed were studied and evaluated for solubility behavior and in-vitro release profile.
Acknowledgements
The author Abdul Wahab is thankful to Higher Education Commission (HEC) of Pakistan for awarding PhD scholarship.
References
- Elkordy AA, Ebtessam AE. Dissolution of Ibuprofen from Spray Dried and Spray Chilled Particles. Pak J Pharm Sci. 2010; 23: 284-290. Ref.: https://tinyurl.com/yc9yr2jd
- Chaulang G, Piyush P, Sharwaree H, Mukul K, Ashok B, et al. Formulation and Evaluation of Solid Dispersions of Furosemide in Sodium Starch Glycolate. Trop J Pharm Res. 2009; 8: 43-51. Ref.: https://tinyurl.com/ybs9bgdr
- Naseem A, Olliff CJ, Martini LG, Lioyd AW. Effects of plasma irradiation on the wettability and dissolution of compacts of griseofulvin. Int J Pharm. 2004; 269: 443-450. Ref.: https://tinyurl.com/y8mvlkuf
- Galia E, Nicolaides E, Horters D, Lobenberg R, Reppasc, et al. Evaluation of various dissolution media for predicting invivo performance of class I & II drugs. Pharm Res. 1998; 15: 698-705. Ref.: https://tinyurl.com/ya2ftbhv
- Noyes AA, Whitney WR. The rate of solution of solid substances in their own solutions. J Am Chem Soc. 1897; 19: 930-934. Ref.: https://tinyurl.com/y9g5rfnk
- Khan GM, Zhu JB. Preparation, characterization, and dissolution studies of ibuprofen solid dispersions using polyethyleneglycol (PEG), talc, and PEG-talc as dispersion carriers. Drug Dev Ind Pharm. 1998; 24: 455-462. Ref.: https://tinyurl.com/yd8hbce8
- Damian F, Blaton N, Naesens L. Physicochemical characterization of solid dispersions of the antiviral agent UC-781 with Polyethylene glycol 6000 and Gelucire 44/14. Eur J Pharm Sci. 2000; 10: 311-322. Ref.: https://tinyurl.com/ya4gex7w
- Craig DQ. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm. 2002; 231: 131-144. Ref.: https://tinyurl.com/ycw3phus
- Khan GM, Zhu JB. Dissolution studies of ibuprofen powders: Effect of particle size on dissolution rate and simulation of dissolution process of mixed binary systems. J Chinese Pharm Sci. 1998; 7: 11-17.
- Al-Hamidi H, Edwards AA, Mohammad AA, Nokhodchi A. To enhance dissolution rate of poorly water-soluble drugs: Glucosamine hydrochloride as a potential carrier in solid dispersion formulations. Colloids Surf B Biointerfaces. 2010; 76: 170-178. Ref.: https://tinyurl.com/y8oevafd
- Wahab A, Khan GM, Akhlaq M, Khan NR, Hussain A, et al. Formulation and Evaluation of Controlled Release matrices of Ketoprofen and influence of different Co-excipients on the Release Mechanism. Pharmazie. 2011; 66: 677-683. Ref.: https://tinyurl.com/y9tz8mdg
- Hussain A, Khan GM, Shah SU, Shah KU, Rahim N, et al. Development of a novel ketoprofen transdermal patch: effect of almond oil as penetration enhancer on in-vitro and ex-vivo penetration of ketoprofen through rabbit skin. Pak J Pharm Sci. 2012; 25: 227-232. Ref.: https://tinyurl.com/y85zp5qs
- Parsad KA, Narayanan N, Rajalakshmi G. Preparation and Evaluation of Solid Dispersion of Terbinafine Hydrochloride. Int J Pharm Sci Rev Res. 2010; 3: 130-134. Ref.: https://tinyurl.com/yap9noea
- Jain R, Jain Kaushal, Setty CM, Patel D. Preparation and Evaluation of Solid Dispersions of Aceclofenac. Int J Pharm Sci Drug Res. 2009; 1: 32-35.
- Rosario P, Maranilla F, Giovanni P. Preparation of solid dispersions of nonsteroidal anti-inflammatory drugs with acrylic polymers and studies on mechanisms of drug-polymer interactions. AAPS PharmSciTec. 2002; 3: 10. Ref.: https://tinyurl.com/ydbqr5o9
- Khan GM, Jiabi Z. Formulation and In-vitro Evaluation of Ibuprofen-carbopol 974-NP Controlled-release Matrix Tablets. III: Influence of Co-excipients on the Release Rate of the Drug. J Control Release. 1998; 54: 185-190. Ref.: https://tinyurl.com/ycn69cqr
- Nagarsenkar MS, Hira S. Influence of Hydroxypropyl β-Cyclodextrin on Solubility and Dissolution Profile of Ketoprofen in Its Solid Dispersions. Drug Development and Industrial Pharmacy. 1996; 22: 9-10. Ref.: https://tinyurl.com/ya963cxb
- Sancin P, Caputo O, Cavallari C, Passerini N, Rodriguez L. Effects of ultrasound-assisted Compaction on Ketoprofen/ Eudragit S100 Mixtures. Eur J Pharm Sci. 1999; 7: 207-213. Ref.: https://tinyurl.com/yb9nlrvp
- Mura P, Bettinetti GP, Manderjoli A, Favcci MT, Bramanti G, et al. Interactions of ketoprofen and Ibuprofen with β-cyclodextrins, in Solution and in the Solid State. Int J Pharm. 1998; 166: 189-203. Ref.: https://tinyurl.com/ya3rnagq
- Shivakumar HN, Sarasija S, Desai BG. Design and Evaluation of Cotrolled onset extended release multiparticulate systems for chronotherapeutic delivery of ketoprofen. Indian J Pharm Sci. 2008; 66: 76-82. Ref.: https://tinyurl.com/y9fxeyko
- Ford JL. The current status of solid dispersions. Pharm Acta Helv. 1986; 61: 69-88. Ref.: https://tinyurl.com/ybvjg5t4
- Martinez-Oharriz MC, Rodrig-Espinosa C, Martin C, Goni MM, Trosllarduya MC, et al. Solid dispersions of diflunisal-PVP: Polymorphic and amorphous states of the drug. Drug Dev Ind Pharm. 2002; 28: 717-725. Ref.: https://tinyurl.com/y7c9ukkh