Research Article
Preclinical studies for a cationic liposome formulation containing Il-2 Intended for the treatment of Human Tumors

Maria Teresa Corona-Ortega1*, Arturo Valle-Mendiola1, Leonor Aguilar-Santelises2, Araceli Garcia del Valle2, Rosalva Rangel-Corona1 and Benny Weiss-Steider1
1FES-Zaragoza, National Autonomous University of Mexico, Mexico City, Lab. 4 P.B. UMIEZ Campo II. Apartado Postal 9-020 Mexico, 15000, D.F. Mexico City, México
2FES-Zaragoza, National Autonomous University of Mexico, Mexico City, Pharmaceutical Manufacturing Plant, Campo II. Apartado Postal 9-020 México, 15000, D.F., Mexico City, Mexico
*Address for Correspondence: María Teresa Corona-Ortega, FES-Zaragoza, National Autonomous University of Mexico, Mexico City, Lab. 4 P.B. UMIEZ Campo II. Apartado Postal 9-020 México, 15000, D.F., Mexico City, Mexico, Tel / Fax: (52) 55- 5773-4108; Email: [email protected]
Dates: Submitted: 10 October 2018; Approved: 26 October 2018; Published: 29 October 2018
How to cite this article: Corona-Ortega MT, Valle-Mendiola A, Aguilar-Santelises L, del Valle AG, Rangel-Corona R, et al. Preclinical studies for a cationic liposome formulation containing Il-2 Intended for the treatment of Human Tumors. Arch Pharm Pharma Sci. 2018; 2: 051-059. DOI: 10.29328/journal.apps.1001010
Copyright License: © 2018 Corona-Ortega MT, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Human cervical cancer tumours expressing the IL-2 receptor (IL-2R) were induced in the peritoneal cavity of nude mice. The tumours were significantly reduced by the i.p. administration of either free IL-2 or liposomes containing this growth factor. No toxicity was observed in the mice even at the highest doses of IL-2 in liposomes. We did not detect any IL-2 in the blood plasma pointing to a strong retention of the liposomes on the cavity. We concluded that this preclinical study for the treatment of tumours expressing IL-2R in the peritoneal cavity is effective and safe. The liposomes were stable and their IL-2 active for up to one year when kept at -14oC in a cryopreservation media approved by the FDA for human use.
Introduction
Tumors can occur in the peritoneal cavity either as primary or metastatic growths [1-7]. Besides the lymphatic and hematologic routes of dissemination the transcoelomic spread of tumor cells is an acknowledged phenomenon that ultimately leads to peritoneal carcinomatosis [8,9]. The administration of anticancer drugs in the peritoneal cavity suffers from a rapid clearance by the lymphatic system into blood circulation and by its absorption in the large surface of the peritoneum [10,11]. Liposomes have been widely studied as drug carriers [12-16] and shown that when injected i.p. their retention in the cavity is significantly increased [17-19].
IL-2 has been used to treat human tumors with relative success. Nevertheless the high doses needed for an effective antitumor effect is rather toxic thus limiting the use of this molecule in cancer therapy [20]. The high toxicity associated with IL-2 has been shown to be due to the cytokines released by T lymphocytes activated by this growth factor [21]. The i.p. administration of IL-2 has been shown to be mostly conserved in the cavity with only slight increase in plasma or organs [22].
Lower doses of IL-2 cannot be used in cancer treatment because on one hand they do not activate cytotoxic lymphocytes and on the other it has been shown that they promote tumor growth [23]. We have previously shown that high doses of IL-2 can be included in liposomes with significant antitumor activity without the high toxicity produced by free IL-2 [24]. We used a chemical immune depressed mouse model to facilitate the formation of human cervical cancer tumors that express the IL-2R. The cationic liposome developed by our group contains the IL-2 molecules presented on its external surface thus facilitating their recognition by cells expressing the IL-2 receptor [25]. Several cancer cells have been shown to express IL-2R [26-29], thus susceptible to be treated by these liposomes.
This work was undertaken to evaluate the effectiveness and safety of our liposomes as an anticancer drug in vivo by using a nude mouse model. The advantage of this model is that human tumors can be produced without immune rejection and in our particular case because IL-2 being a lymphocyte growth factor could not excerpt indirectly its antitumor activity by activating cytotoxic T cells. An additional advantage being that the animals were not chemically immune depressed thus avoiding the inherent systemic toxicity. In our previous model the existence of a partially functional immune system generated significant organ damage when free IL-2 was used. We expected that in the nude model the absence of thymic lymphocytes this toxicity could be avoided.
We reasoned that if liposomes increase the permanence of associated drugs in the peritoneal cavity and if IL-2 by itself is known to remain in that cavity a liposome containing this growth factor could have an important cavity stay. On the other hand if tumors bearing the IL-2R are known to strongly deplete IL-2 from the surrounding media then the administration a liposomes presenting external IL-2 by this via can further avoid its entrance into blood circulation augmenting its antitumor activity in the cavity itself and eliminating the systemic tumor promoting effect associated with low doses of IL-2.
Our results showed a strong anticancer effect of these liposomes without any mice toxicity and the absence of plasma IL-2 even at very high doses. We consider this work to be a successful preclinical study by showing the effectiveness and safety of our liposome for treating peritoneal tumors expressing the IL-2R.
Materials and Methods
Reagents
Human recombinant Interleukin 2 (rhIL-2) was acquired from R&D systems (Minneapolis, MN, USA) and was reconstituted according to the supplier’s instructions. Egg yolk phosphatidyl-choline (PC) and cholesteryl-spermidine (SpeCho), synthesized by direct reaction of cholesteryl chloroformate with spermidine base, which were acquired from Sigma (Sigma Chem, St Louis, MO, USA). Cryopreservation agents were acquired too from Sigma (Sigma Chem, St Louis, MO, USA).
Preparation and storage of liposomes
Cationic liposomes were formed as published by our group [25] using the cationic lipid Cholesteryl-spermidine (SpeCho) (synthesized in our laboratory) and egg yolk phosphatidylcholine (PC), (Sigma Chem, Mo. USA) in a 1:1 molar ratio. The mixture of lipids (10 µmol) was dissolved in chloroform dried under nitrogen at reduced pressure and liposomes produced by hydration of the thin lipid film in phosphate buffer solution (PBS) with or without 100IU/mL of IL-2 (2.4 X 103 IU of IL-2 is equivalent to 1 µG of IL-2) in PBS using three 5 second sonication cycles followed by 30 seconds resting period in an Avanti G112SO20_B sonicator. The liposomes were finally sedimented at 200,000g for 40 minutes in PBS and suspended in a solution that containing a mixture of cryopreservation agents (trehalose/glycerol) authorized by the FDA for human use.
Samples with 300 µL were used for our experiments or kept frozen at -14oC for stability evaluation.
Transmission electron microscopy
The liposome suspensions with or without IL-2 were diluted 1:1 with 4% aqueous uranyl acetate on carbon-Formvar-coated grids and incubated for 1 min. The grids were drained on absorbent tissue and allowed to air-dry. A JEOL-JEM-1010 transmission electron microscope (JEOL USA, Inc., USA) was used.
Flow cytometry
Liposomes in 100 µL of PBS with or without IL-2 were analyzed in a FACS Aria flow cytometer (Becton Dickinson, CA, USA). Results were reported as laser beam diffraction (FSC) taken to be proportional to liposomes surface area (size) or refraction (SSC) taken as their complexity (roughness).
Cell culture
A human solid tumor cell line INBL established in our laboratory from a cervical carcinoma was kept in tissue culture and routinely sub-cultured at 37ºC with 5% CO2 in RPMI 1640 medium (Microlab, México) supplemented with 10% fetal calf serum (FBS) (Hyclone, USA).
Animals
Athymic nu/nu female mice between 8 and 12 weeks old were used. Mice were kept in groups of three on sterile environment in cages at room temperature with food and water ad libitum as per the protocols approved by the UNAM’s Animal Experimentation and Ethics Committee which complies with the code of practice regarding the care and use of animals for scientific purposes.
Tumor induction
Mice were inoculated i.p. with 8x106 INBL cells in 300 μl of PBS. The animals were kept for 20 more days for the different antitumor protocols to be applied. Subsequently sacrificed by cervical dislocation and the tumor masses collected. The volumes were calculated assuming they had a spherical shape by using the formula 4/3 πr3 where r was half of their longest diameter.
Toxicology assay
The toxicology assay was done according to Lorke Model [30], for increasing concentrations of IL-2. Liposomes were administered i.p. in a single dose with 100, 500 and 5000 IU of IL-2. Mice were observed for 15 days and blood samples collected in Microtainer® microtubes (BD Diagnostics, Franklin Lakes, NJ, USA) and the serum separated by 15 min of centrifugation at 350 g at 4ºC. The amount of urea, creatinine, transaminases AST and ALT were then evaluated.
Pharmacodynamic assay
For the pharmacodynamic assay we induced tumor formation, as described in Materials and Methods, in three groups of three mice by triplicate. Once the tumors were established we administrated i.p. 100 IU/mL of IL-2 either free or in liposomes diluted in 300 μL on a daily basis for 5 days. A group of mice with only the dilution media was used as a control. The animals were then sacrificed by cervical dislocation and the tumor masses collected.
Analytical determination of rhIL-2 in serum
The analytical determination of rhIL-2 content in serum was done with a validated analytical assay using an ELISA sandwich. Briefly; 100 μl/well of capture antibody (MAB 602, R&D Systems, Minneapolis, MN, USA) were added in a 96 well ELISA plate (Sarstead, Nümbrecht, GER) for 24 hours at room temperature followed by three washes with 0.05% Tween 20 in PBS at pH 7,4 and then with 1% polyvinylpyrrolidone (PVP) for one hour to block empty spaces. After one more wash cycle of 100 μl/well serum samples were added and incubated for 2 more hours. After one more wash 100 μL of the biotinylated detection antibody (BAF 202, R&D Systems, Minneapolis, MN, USA) were added and incubated for 2 hours at room temperature. After two more washing cycles 100 μL of Ultrasensitive Streptavidin-HRP (Sigma Chem, St Louis, MO, USA) diluted according to supplier’s instructions were added to each well. The plate was then covered and incubated for 30 minutes at room temperature, avoiding placing the plate under direct light. After one more wash cycle 100 μL of a TMB solution (Sigma Chem, St Louis, MO, USA) diluted according to the supplier´s instructions was added to each well. The plates were then incubated for 30 minutes at room temperature, avoiding placing the plate under direct light. 50 μL of 1 M H2SO4 were added to each well and with a gentle tap to the plate to ensure thorough mixing the optical density (O.D.) at 450 nm was determined for each well within 30 minutes with a wavelength correction to 570 nm.
Results
Pharmacodynamics measured as an antitumor effect of liposomes in tumors induced by a human cervical cancer cell line in nude mice
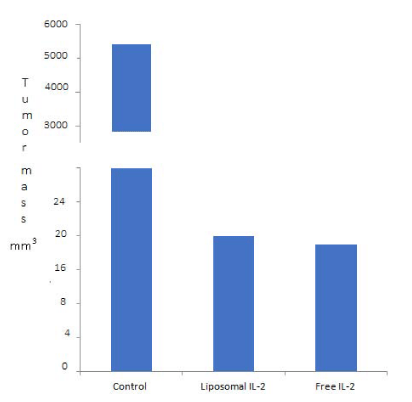
We have previously demonstrated that 100 UI/Ml of IL-2 in our liposomes had a strong antitumor effect on an immunodepressed CBA mice model bearing human cervical cancer tumors [24]. In this work we used nude mice (nu/nu) to avoid the toxicity involved in the previously used immune depression protocol. For this purpose we induced tumors in three groups with three nude mice each with the human cervical cancer cells INBL as described in Materials and Methods. After 20 days of tumor induction two groups were inoculated for five consecutive days with our liposomes diluted in trehalose/glycerol with 100 UI/Ml of IL-2 or with the same concentration of free IL-2. The third group was left without treatment as a negative control. The mice were then sacrificed and the volume of total tumor masses evaluated. As expected we obtained large tumor masses in the control group (5082 mm3) while more that 95 % reduction with our liposomes (16 mm3) and (15 mm3) with free IL-2 (Figure 1).
Figure 1: Tumor volume induced in mice. Control (5082mm3), liposomes (16 mm3) and IL-2 free (15 mm3). Differences between control and treatments are significative.
Toxicity of liposomes containing different concentrations of IL-2 in nude mice
We have previously obtained that the maximum load of IL-2 in our liposomes was of 5000 IU/ML [31], in this work we evaluated toxicity of 100, 1000 and 5000 IU/mL of IL2 in liposomes dissolved in trehalose/glycerol in our nude mouse model as evaluated by their urea, creatinine, transaminases AST and ALT content. For this purpose three groups of three nude mice were inoculated i.p. with 300 µL of the different IL-2 concentrations in liposomes. Control groups with either no treatment, with only vehicle (trehalose/glycerol) or with liposomes without IL-2 were used. Mice were observed daily for 15 days and then sacrificed to obtain blood serum. No dizziness, fever, vomit, convulsions, tachycardia or any other behavior change was observed except for a slight irritation towards noise at the highest dose. Our results showed no departures from the mice reference values [32] or from those of controls in the amount of creatinine, urea, AST or ALT in their sera (Table 1).
| Table1:Levels of creatinine, urea, AST and ALT in sera of nu/nu mice after 15 days of inoculated with different doses of IL-2 in liposomes. There are no significative differences because all values reside between the reference and control ones. | |||||||
| Parameter | Mice references values | Control without treatments | Vehicle | Liposomes without IL-2 | Liposomes containing 100UI/mL | Liposomes containing 1000UI/mL | Liposomes containing 5000UI/mL |
| Urea (mg/dL) | 41.4 - 47.5 | 60.5+/- 3 | 40.8 +/- 4 | 60.3 +/- 5 | 42.8 +/- 5 | 39.6+/- 3.5 | 41 +/- 4 |
| Creatinin (mg/dL) | 0.51–0.55 | 0.2 +/- 0.01 | 0.3 +/- 0.09 | 0.2 +/- 0.0 | 0.2 +/- 0.01 | 0.2 +/- 0.04 | 0.2 +/- 0.0 |
| AST (U/L) | 55-251 | 164.3 +/- 26 | 193.5 +/- 35 | 264 +/- 15 | 280.3 +/- 94 | 288 +/- 74 | 213.6 +/- 19 |
| ALT (U/L) | 28-184 | 35.5 +/- 7 | 32 +/- 0.0 | 47 +/- 23 | 43 +/- 11 | 53 +/- 14 | 48 +/- 31 |
No IL-2 activity was found in the sera of nude mice treated with liposomes
To evaluate the possible presence of IL-2 in plasma we used two groups of 24 nude mice. We administered i.p. on each one either a single dose of free 100 IU/mL of IL-2 or liposomes with the same concentration of IL-2 in 300 μL of trehalose/glycerol. After 0, 10, 20, 30 60 and 120 min two animals of each group were anesthetized and blood collected. No IL-2 activity was detected in any of the sera.
Stability of liposomes containing IL-2 in the cryopreservation mixture containing trehalose and glycerol
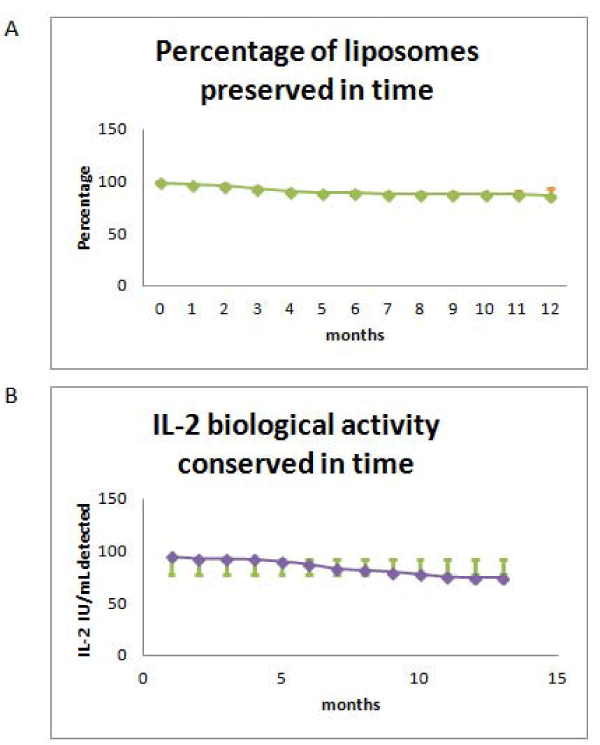
The activity of IL-2 in our liposomes became rapidly inactivated, as the provider stated, at room temperature and 4oC. In order to evaluate if the liposomes could be kept active when frozen for long periods of time we calibrated our freezer and found that the temperature of -14oC was constant at the central shelves and decided to use them for our experiments. We obtained that while the IL-2 activity in our liposomes was conserved at this temperature the liposome structure was destroyed. In order to avoid liposome destruction we substituted PBS for a combination of trehalose and glycerol recommended by the FDA [33-35], for human application and evaluated IL-2 activity and liposome preservation as a function of time at -14oC. For that purpose we froze 36 samples and every month three samples were unfrozen and evaluated up to one year. We obtained that the activity and structure stability of the liposomes were mostly conserved (Figure 2A,B).
Figure 2: Stability of frozen liposomes containing IL-2 as a function of time. A: Integrity evaluated by flow cytometry as the percentage size of liposomes as compared to the control that was taken as 100%; B: Percentage of biological activity of IL-2 contained in liposomes evaluated by an analytical assay as compared to a control taken as 100%. Media: Trehalose/glycerol; CV: variation coefficient.
Increase in size and roughness of liposomes in the cryopreservation mixture
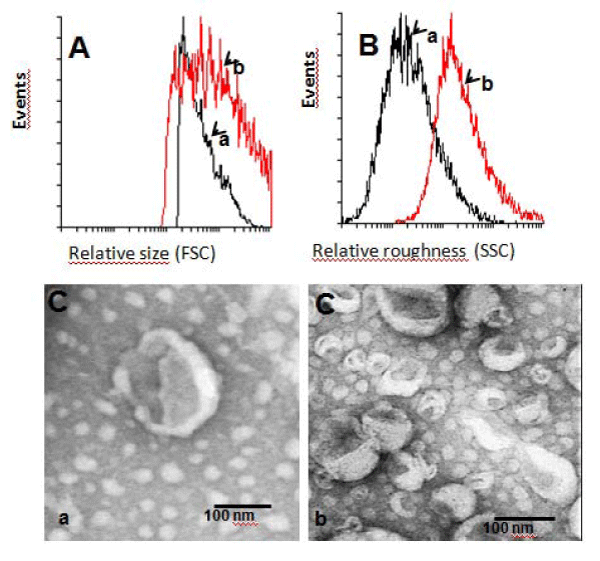
Once we determined that the IL-2 activity in our liposomes did not change significantly even after freezing for up to one year hinting to encapsulation efficiency, the size and shape of liposomes either diluted in PBS or in a trehalose/glycerol mixture were compared by transmission electron microscopy and FACS analysis. We obtained that the size and roughness of the liposomes in the cryopreserved mixture increased significantly (Figure 3A,B).
Figure 3: Size and roughness of liposomes evaluated by flow cytometry and electron microscopy. A: Relative size by flow cytometry; B: Relative roughness by flow cytometry; C: Size evaluated by transmission electron microscopy. a) Liposomes without cryopreserver; b) Liposomes with cryopreserver.
Discussion
The IL-2 receptor (IL-2R) has been found to be express in a variety of epithelial cancer cells. Even though this receptor has been well characterized to activate lymphoid cells, its function on epithelial cells is mostly unknown. It was hypothesized that the IL-2R in cancer cells could on one hand deplete IL-2 from the tumor environment thus escaping attack from lymphocytes that need this factor to become cytotoxic and on the other hand by using this factor for their own proliferation. We have previously used a chemical immune depressed animal model and found a strong antitumor effect of a liposome presenting IL-2 on its external surface. We hypothesized that the strong apoptotic cell death found in the human cervical cancer cells was due to a direct interaction between the IL-2 in the liposomes and the IL-2R on the tumor cells. Nevertheless we could not completely rule out the participation of cytotoxic lymphocytes. The choice of a nude mice model used in this work warranted the formation of a human tumor without the need for chemical immunodepression and eliminating the possible antitumor effect of cytotoxic lymphocytes that could also be activated by the liposome´s IL-2.
The peritoneal cavity was chosen to induce the tumors because it is known that administrated liposomes remain mostly on the cavity with almost no lymphatic drainage. We obtained a very strong antitumor activity with more than 90% volume reduction when treated with our liposomes and we did not detect any IL-2 on the mice sera even after 120 min of liposome administration. It was important to avoid the presence of IL-2 in blood because of the known toxic systemic side effects that might be present when treating human cancers.
The peritoneal cavity has been found to be the site of secondary tumors by infiltrating malignant cells or by overgrowth of primary tumors. Once tumors grow in the cavity the patient prognosis is poor especially in those cases with advanced stages. Liposomes containing antitumor chemicals have been used to treat these tumors with limited success. In this work we chose to grow a tumor in the peritoneal cavity to try to find a therapeutic alternative to these types of cancers.
This preclinical study shows that the liposome has a strong anticancer effect in vivo with no toxicity thus safe for human use. It would be interesting to evaluate the doses and regimes needed to eradicate the tumor completely and thus the kinetics of IL-2 in the cavity as a function of time. Another advantage of our liposomes is that when applied into human cancers the absence of systemic IL-2 avoids the toxicity associated with this growth factor [20,21]. In this respect the liposome structure and activity of the IL-2 molecule was mostly conserved for up to one year at -14oC in a cryopreserver media approved by the FDA for human use.
We cannot discard the possible application of our liposome for primary or secondary tumors that do not express the IL- 2R because the IL-2 they contain could also activate residual lymphocytes to become cytotoxic. In this respect it has been shown the existence of inactivated T lymphocytes in the peritoneal cavity that can be induced to become [36,37]. Finally we think that this technique can also be useful in other serous membranes tumors like those in the pericardial and pleural cavities that in general have poor prognosis [38,39].
Acknowledgement
The authors acknowledge support from a DGAPA, UNAM IN219315 and IN 216018 grants. We thank Dr. Luis Felipe Jiménez García and Dr Reyna Lara Martínez from Tlahuizcalpan Laboratory for electron microscopy, Facultad de ciencias UNAM, QFB Berenice Campos Castilla for assistance with image and data processing and QFB Manuel Martin Calderón Martínez for the careful review and comments for the pharmacokinetic data.
References
- Spottswood SE, Lopatina OA, Fey GL, Boardman CH. Peritoneal carcinomatosis from cervical cancer detected by F-18 FDG positron emission tomography. Clin Nucl Med. 2005; 30: 56-59. Ref.: https://goo.gl/PehsiG
- Bekes I, Friedl TW, Köhler T, Möbus V, Janni W, et al. Does VEGF facilitate local tumor growth and spread into the abdominal cavity by suppressing endothelial cell adhesion, thus increasing vascular peritoneal permeability followed by ascites production in ovarian cancer? Mol Cancer. 2016; 15: 13. Ref.: https://goo.gl/wg6wjn
- Auer K, Bachmayr-Heyda A, Aust S, Sukhbaatar N, Reiner AT, et al. Peritoneal tumor spread in serous ovarian cancer-epithelial mesenchymal status and outcome. Oncotarget. 2015; 6: 17261-17275. Ref.: https://goo.gl/8ePYYe
- Kurihara H. Integrated 64Cu therapy for the peritoneal dissemination of gastrointestinal cancer. Oncotarget. 2018; 9: 31165-31166. Ref.: https://goo.gl/WTKXtq
- Sugarbaker PH. Peritoneal Metastases from Gastrointestinal Cancer. Curr Oncol Rep. 2018; 20: 62. Ref.: https://goo.gl/nr3oy2
- Mugerwa S, Lekharaju V, Kiire CF. Management of peritoneal carcinomatosis secondary to metastatic cancer of unknown primary in men. Eur J Cancer Care (Engl). 2009; 18: 22-27. Ref.: https://goo.gl/hztJmX
- Levy AD, Shaw JC, Sobin LH. Secondary tumors and tumorlike lesions of the peritoneal cavity: imaging features with pathologic correlation. Radiographics. 2009; 29: 347-373. Ref.: https://goo.gl/QeTQjA
- de Bree E, Witkamp AJ, Zoetmulder FA. Intraperitoneal chemotherapy for colorectal cancer. J Surg Oncol. 2002; 79: 46–61. Ref.: https://goo.gl/XksNuC
- Van der Speeten K, Stuart OA, Sugarbaker PH. Using pharmacologic data to plan clinical treatments for patients with peritoneal surface malignancy. Curr Drug Discov Technol. 2009; 6: 72–81. Ref.: https://goo.gl/1gKBxL
- Dadashzadeh S, Mirahmadi N, Babaei MH, Vali AM. Peritoneal retention of liposomes: Effects of lipid composition, PEG coating and liposome charge. J Control Release. 2010; 148: 177-186. Ref.: https://goo.gl/WHQefQ
- Mirahmadi N, Babaei MH, Vali AM, Dadashzadeh S. Effect of liposome size on peritoneal retention and organ distribution after intraperitoneal injection in mice. Int J Pharm. 2010; 383: 7-13. Ref.: https://goo.gl/HJBYpf
- Ulrich AS. Biophysical aspects of using liposomes as delivery vehicles. Bioscience Reports. 2002; 22: 129-150. Ref.: https://goo.gl/MhEH7w
- Fatima MT, Islam Z, Ahmad E, Barreto GE, Md Ashraf G. Ionic gradient liposomes: Recent advances in the stable entrapment and prolonged released of local anesthetics and anticancer drugs. Biomed Pharmacother. 2018; 107: 34-43. Ref.: https://goo.gl/uT2Wck
- Franco MS, Oliveira MC. Liposomes co-encapsulating anticancer drugs in synergistic ratios as an approach to promote increased efficacy and greater safety. Anticancer Agents Med Chem. 2018; Ref.: https://goo.gl/jQRzj2
- Zhang T, Zhou S, Hu L, Peng B, Liu Y, et al. Polysialic acidpolyethylene glycol conjugate-modified liposomes as a targeted drug delivery system for epirubicin to enhance anticancer efficiency. Drug Deliv Transl Res. 2018; 8: 602-616. Ref.: https://goo.gl/nD6mnK
- Jang EJ, Choi WR, Kim SY, Hong SS, Rhee I, et al. 2-Hydroxyoleic acid-inserted liposomes as a multifunctional carrier of anticancer drugs. Drug Deliv. 2017; 24: 1587-1597. Ref.: https://goo.gl/ZLHLG7
- Syrigos KN, Vile RG, Peters AM, Harrington KJ. Biodistribution and pharmacokinetics of 111In-DTPA-labelled pegylated liposomes after intraperitoneal injection. Acta Oncol. 2003; 42: 147–153. Ref.: https://goo.gl/HD54Sc
- Chen LC, Chang CH, Yu CY, Chang YJ, Hsu WC, et al. Biodistribution, pharmacokinetics and imaging of 188Re-BMEDA-labeled pegylated liposomes after intraperitoneal injection in a C26 colon carcinoma ascites mouse model. Nucl Med Biol. 2007; 34: 415–423. Ref.: https://goo.gl/cduMTb
- Zavaleta CL, Phillips WT, Soundararajan A, Goins BA. Use of avidin/biotin–liposome system for enhanced peritoneal drug delivery in an ovarian cancer model. Int J Pharm. 2007; 337: 316–328. Ref.: https://goo.gl/Z473Hd
- Schwartz RN, Stover L, Dutcher JP. Managing toxicities of high-dose interleukin-2. Oncology (Williston Park). 2002; 16(11 Suppl 13): 11-20. Ref.: https://goo.gl/4XLwLh
- Li Y, Strick-Marchand H, Lim AI, Ren J, Masse-Ranson G, et al. Regulatory T cells control toxicity in a humanized model of IL-2 therapy. Nat Commun. 2017; 8: 1762 Ref.: https://goo.gl/LMk6eb
- Donohue JH, Rosenberg SA. The fate of interleukin-2 after in vivo administration. J Immunol 1983; 130: 2203-2208. Ref.: https://goo.gl/Bm4KEo
- Rangel-Corona R, Corona-Ortega T, Soto-Cruz I, López-Labra A, Pablo-Arcos T, et al. Evidence that cervical cancer cells secrete IL-2, which becomes an autocrine growth factor. Cytokine. 2010; 50: 273–277. Ref.: https://goo.gl/sWVxeN
- Rangel-Corona R, Corona-Ortega T, del Río-Ortiz I, Nieves-Ramírez ME, Morán-Bañuelos H, et al. Cationic liposomes bearing IL-2 on their external surface induced mice leukocytes to kill human cervical cancer cells in vitro, and significantly reduced tumor burden in immunodepressed mice. J Drug Target. 2011; 19: 79–85. Ref.: https://goo.gl/N3EMLB
- Corona T, Rangel R, Hernández M, Baeza I, Ibáñez M, et al. Characterization of cationic liposomes having IL-2 expressed on the external surface, and their affinity to cervical cancer cells expressing the IL-2 receptor. J Drug Target. 2009; 17: 496-501. Ref.: https://goo.gl/RDBkVR
- O'Loughlin EV, Pang GP, Noltorp R, Koina C, Batey R, et al. Interleukin 2 modulates ion secretion and cell proliferation in cultured human small intestinal enterocytes. Gut. 2001; 49: 636-643. Ref.: https://goo.gl/PVY4Lt
- Du C, Guan Q, Yin Z, Zhong R, Jevnikar AM. IL-2-mediated apoptosis of kidney tubular epithelial cells is regulated by the caspase-8 inhibitor c-FLIP. Kidney Int. 2005; 67: 1397-1409. Ref.: https://goo.gl/Pevp3T
- Kawami H, Yoshida K, Yamaguchi Y, Saeki T, Toge T. The expression and biological activity of IL-2 receptor on a human pancreas cancer cell line. Biotherapy. 1993; 6: 33-39. Ref.: https://goo.gl/XQReZ5
- Barton DP, Blanchard DK, Wells AF, Nicosia SV, Roberts WS, et al. Expression of interleukin-2 receptor alpha (IL-2R alpha) mRNA and protein in advanced epithelial ovarian cancer. Anticancer Res. 1994; 14(3A): 761-772. Ref.: https://goo.gl/GmZai2
- Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983; 54: 275-287. Ref.: https://goo.gl/LG245s
- Corona-Ortega MT, Soto-Vázquez R, Rangel-Corona R, Huante-García RM, AguilarSantelises L, et al. A Novel Nanocarrier System for Cancer Treatment. Current Nanomedicine. 2016; 6: 133-145. Ref.: https://goo.gl/ZrCCe2
- Fernández I, Peña A, Del Teso N, Pérez V, Rodríguez-Cuesta J. Clinical Biochemistry Parameters in C57BL/6J Mice after Blood Collection from the Submandibular Vein and Retroorbital Plexus. J Am Assoc Lab Anim Sci. 2010; 49: 202-206. Ref.: https://goo.gl/NDmvHi
- Kallerup RS, Madsen CM, Schiøth ML, Franzyk H, Rose F, et al. Influence of trehalose 6,60-diester (TDX) chain length on the physicochemical and immunopotentiating properties of DDA/TDX liposomes. Eur J Pharm and Biopharm. 2015; 90: 80– 89. Ref.: https://goo.gl/6TQMWK
- Sydykov B, Oldenhof H, de Oliveira Barros L, Sieme H, Wolkers WF. Membrane permeabilization of phosphatidylcholine liposomes induced by cryopreservation and vitrification solutions. BBA – Biomembranes. 1860, 2018; 467–474. Ref.: https://goo.gl/DHty55
- Corona-Ortega MT, Soto-Vázquez R, Weiss-Steider B, Rangel-Corona R. Inv.: PROCESO DE ESTABILIZACIÓN DE LIPOSOMAS QUE CONTIENEN IL-2 MEDIANTE CRIOPRESERVACIÓN, Patent request, Mx/a/2015/013539.
- Hansson J, Ericsson PO, Dohlsten M, Sjögren HO, Kalland T, et al. Locally superantigen-activated peritoneal cytolytic T lymphocytes belong to the CD8+ CD45RC- subset and lyse MHC class II+ tumor cells. Immunol Lett. 1992; 34: 229-236. Ref.: https://goo.gl/p3aLxf
- Berek JS, Bast RC Jr, Lichtenstein A, Hacker NF, Spina CA, et al. Lymphocyte cytotoxicity in the peritoneal cavity and blood of patients with ovarian cancer. Obstet Gynecol. 1984; 64: 08-14. Ref.: https://goo.gl/btzWMv
- Sakaguchi H, Ishida H, Nitanda H, Yamazaki N, Kaneko K, et al. Pharmacokinetic evaluation of intrapleural perfusion with hyperthermic chemotherapy using cisplatin in patients with malignant pleural effusion. Lung Cancer. 2017; 104: 70-74. Ref.: https://goo.gl/dXmM4Q
- Cao S, Jin S, Cao J, Shen J, Hu J, et al. Advances in malignant peritoneal mesothelioma. Int J Colorectal Dis. 2015; 30: 1-10. Ref.: https://goo.gl/UpMxhz