More Information
Submitted: 29 November 2019 | Approved: 10 December 2019 | Published: 11 December 2019
How to cite this article: Mensah JK, Ibrahim A, Jibira Y. Co-extract mixture from Strophanthus hispidus (roots) and Aframomum meleguta (seeds) show phytochemical synergy in its anti-inflammatory activity. Arch Pharm Pharma Sci. 2019; 3: 089-100.
DOI: 10.29328/journal.apps.1001019
ORCiD: orcid.org/0000-0002-1232-7217
Copyright License: © 2019 Mensah JK, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Strophanthus hispidus; Aframomum meleguta; Anti-microbial; Anti-oxidant; Anti-inflammation
Co-extract mixture from Strophanthus hispidus (roots) and Aframomum meleguta (seeds) show phytochemical synergy in its anti-inflammatory activity
John Kenneth Mensah1*, Amina Ibrahim1 and Yakubu Jibira2
1Department of Chemistry, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana
2Department of Pharmacognosy, Faculty of Pharmacy & Pharmaceutical Sciences, College of Health Sciences, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana
*Address for Correspondence: John Kenneth Mensah, Department of Chemistry, Kwame Nkrumah University of Science and Technology (KNUST), Kumasi, Ghana, Tel: +233 200 693323; Email: [email protected]
Background: Combination of extracts from multiple plants are typically used in ethnomedicine to putatively offer more potent chemotherapeutic and chemopreventive effects than that of individual extracts from single plants. Aqueous extracts from two multipurpose plants Strophanthus hispidus (roots) and Aframomum meleguta (seeds) are topically co-administered in the nasal cavities for the ethnomedicinal management of chronic sinusitis.
Aim: This study assessed the potential phytochemical synergy between constituent extracts of Strophanthus hispidus (roots) and Aframomum meleguta (seeds) in its anti-inflammation, anti-microbial and anti-oxidant effects.
Methods and Materials: Broth dilution assay assessed anti-microbial activities. DPPH radical scavenging assay examined the scope of anti-oxidant activities and inhibition of carrageenan-induced 7-day old chick feet oedema revealed anti-inflammatory activities.
Results: Anti-microbial activities of individual plant extracts in broth dilution assay showed comparable potency to that of the co-extract mixture. Similarly, individual extracts showed levels of DPPH radical scavenging activities in anti-oxidant assay that was comparable to those found for the co-extract mixture. In contrast to these two effects, inhibition of carrageenan-induced 7-day old chick feet oedema revealed an anti-inflammatory activity evoked by co-extract mixtures that was greater than the sum of the individual potencies of the two extracts.
Conclusion: The potential phytochemical synergy of the two plants extracts in its anti-inflammatory response largely validates ethnomedicinal practice and generally confirms growing literature reports that ascribe the net pharmacological activities of herbal extracts to the combined multi-activities of unique phytochemical entities at multiple target sites.
Ethnomedicinal-based management of many diseases often rely on concoctions of multiple plant extracts mixed in permissive co-extract ratios that exploits the unique biological activities of distinct phytochemical pools through additive or synergistic interactions [1-5]. Aqueous extracts from the two botanicals Strophanthus hispidus (roots) and Aframomum meleguta (seeds) has represented a local example of this approach since antiquity and their simultaneous topical co-administration offers effective chemopreventive and chemotherapeutic ethnomedicinal strategy against chronic sinusitis.
Chronic sinusitis is an airway disease characterized by persistent inflammation and microbial infection of the nasal and sinus mucosa. The condition has a prolonged clinical course and many of its current treatment modalities, including conventional medical and surgical therapies are largely ineffective. The observation of an efficacious multi-plant extract ethnomedicinal remedy for chronic sinusitis suggested the possibility that the total pool of extract phytochemicals from the combined Strophanthus hispidus (roots) and Aframomum meleguta (seeds) extracts interact functionally to produce either additive or synergistic chemopreventive and chemotherapeutic effects that may be anti-microbial, anti-oxidant and anti-inflammatory [6]. Therefore, the central hypothesis underlying this work is that extract phytochemicals derived from Strophanthus hispidus (roots) and Aframomum meleguta (seeds) function in biochemical synergy to confer anti-microbial, anti-oxidant and anti-inflammatory effects. This key hypothesis that potential phytochemical synergy between constituent extract account for its robust effect against chronic sinusitis was therefore examined by the experimental assessment of the anti-microbial, anti-oxidant and anti-inflammation bioactivities of both individual extracts and co-extract mixture.
Both Strophanthus hispidus (roots) and Aframomum meleguta (seeds) are botanicals endogenous to the tropical Africa and each plant finds extensive use, alone or in combination with other plants, in prevalent ethnomedicinal cultures of several African states [4,7-13]. Both Strophanthus hispidus (roots) and Aframomum meleguta (seeds) have been systematically lab-tested for anti-microbial, anti-oxidant and anti-inflammatory effectiveness [7-13]. However, the possible synergistic or additive response that is evoked in their phytochemical bioactivities when combined in ethnomedicinal remedy has not been scientifically elucidated. Consequently, there is little current evidential scientific support for the historical and enduring ethnomedicinal utilization of the combined extracts of Strophanthus hispidus (roots) and Aframomum meleguta (seeds) for the clinical management of chronic sinusitis.
Using this ethnomedicinal knowledge base as pharmaco-logical support and the methanol extracts of both plants as phytochemicals sources, this study presents evidence indicative of synergistic interaction of Strophanthus hispidus (roots) and Aframomum meleguta (seeds) phytochemicals to the anti-inflammation activities of the co-extract. The study shows that co-administered topically, co-extract phytochemicals inhibit microbial growth, scavenge free radicals and their complementary physiological effects synergizes to inhibit inflammation. Taken together, the results indicate that co-extract anti-inflammatory bioactivity may derive from functional synergism between the phytochemical pools of the two plant extracts. Interestingly, this synergistic effect was not observed for the anti-microbial and anti-oxidant bioactivities.
Although the exact mechanistic action and mechanistic targets of individual extract phytochemicals are unknown, evidence from this study suggests that the ethnomedicinal application of the co-extracts in the management of sinusitis may have scientific merit. This study fills an important mechanistic void in ethnomedicine with its suggestion of a synergistic induction of in vivo anti-inflammation activity by phytochemical combinations from Strophanthus hispidus (roots) and Aframomum meleguta (seeds).
Extracts of Strophanthus hispidus (roots) and Aframomum meleguta (seeds) were individually examined for anti-microbial, anti-oxidant and anti-inflammatory effects and then aliquots of both extracts blended in a 3:2 (weight/weight) ratio as the co-extract synergy mixture was similarly assessed for anti-microbial, anti-oxidant and anti-inflammatory effects.
Plant materials
Strophanthus hispidus (roots) and Aframomum meleguta (seeds) were selected for this study because of their historically high use prevalence and their reputed ethnomedicinal efficacy. Plant specimens were purchased in the local market and purchased plants were verified in terms of genus and species at the Department of Biology of KNUST. Voucher plant specimens were retained at the school of Pharmacy of KNUST.
Soxhlet extraction
Methanol extracts of (Strophanthus hispidus (roots) and Aframomum meleguta (seeds) were obtained by Soxhlet extraction as previously described [14]. Extracts were concentrated on a rotavap and the resulting residue stored at -20 ˚C until needed. Stock solutions of 20 mg/mL of (Strophanthus hispidus (roots) extract and of Aframomum meleguta (seeds) extract were prepared in 99% methanol and diluted as needed for different assays. Diluted extracts solutions were filter sterilized before use.
Regional or geographical differences in plant location, subtle genotypic differences in plant genomes as well as seasonal harvest differences in plant phytochemical content are known to affect the chemotype and the quantity of extract phytochemicals. Consequently, experimental extracts could not be standardized with any known reference standard. Neither the acute nor the chronic toxicity of extracts were examined in this study.
TLC
Silica gel thin-layer chromatography (TLC) was employed to estimate the approximate number of distinct chemical entities within each extract. Briefly, a small sample of the stock extract solutions were dissolved in 1 mL methanol and spotted on an in-house prepared 10 cm by 5 cm and 0.2 mm thick silica gel plate as previously described [14]. Developing solvent system utilized for the separation of constituents was ethyl acetate and petroleum ether in a 4:1 ratio. Iodine vapor visualization of resolved chromatographic bands and calculation of Rf values of constituent bands were performed as described earlier [14].
Phytochemical analyses
Phytochemical composition of each crude extracts was assessed using slight modifications of the methods of Trease and Evans [15] as previously described [16].
Synergy extract mixture and synergy studies
To assess the potential phytochemical synergy in inhibitory effects toward microbial growth, ROS production and inflammation, a 3:2 (weight/weight) Strophanthus hispidus (roots): Aframomum meleguta (seeds) extracts ratio was used. Pegging of the co-extract synergy mixture ratio at 3:2 (weight/weight) was based on best practices guidelines observed by local herbalists who rely on the phytochemical mixture of the two botanicals for the ethnomedicinal management of sinusitis. Utilization of this weight ratio implies that final co-extract concentrations possesses reduced individual extracts concentrations relative to that of either extract alone.
Synergy studies
The prevailing hypothesis undergirding this work was that combined extract phytochemicals from (Strophanthus hispidus (roots) and Aframomum meleguta (seeds) will produce synergistic activity. Potential functional synergism by co-extract phytochemicals will be observed through quantitative differences in their molecular actions in anti-microbial, anti-oxidant and anti-inflammatory assays. Consequently, individual extracts were first examined singly and then in a combination maintained at the 3:2 (Strophanthus hispidus (roots) and Aframomum meleguta (seeds) (w/w) ratio. Synergy was quantitatively taken to be the bioactivity of the co-extract mixture that was greater than the sum of the individual bioactivity of either extract alone while antagonism was defined as the co-extract bioactivity that was lower than the sum of individual bioactivity of each extract.
Anti-inflammatory assay
The Carrageenan-induced foot oedema in 7-day old chick model was used for the assessment of the anti-inflammatory effects of extracts and of the co-extract synergy mixture. Chicks were randomly divided into groups of five and a 1% suspension of carrageenan in distilled water was administered as 0.1 ml subplantar injection into the footpad of the right foot as previously described [14]. Chicks were then treated intraperitoneally with vehicle or with extracts (30, 100, 300 mg/kg body weight) or with co-extracts (3, 10 mg/kg body weight) in vehicle. The negative control animals received only the vehicle while a positive control group received reference drugs dexamethasone (0.3, 1, and 3 mg/kg body weight) and diclofenac (10, 30, and 100 mg/kg body weight). Foot volumes were checked before carrageenan injection and at hourly intervals for five hours as described [14]. Oedema volumes were determined as the difference between the foot volumes of each chick before carrageenan injection and the foot volumes at the hourly time intervals.
Data analysis utilized a one-way analysis of variance and differences between groups of chicks analyzed by Bonferroni’s modified t test. A p value of 0.05 was considered significant. Extract or control drug treatment-mediated decreases in the volumes of oedema were utilized as a quantitative measure of the anti-inflammatory response. Post-treatment hourly estimated oedematous volumes was used as a graphical time course representation of anti-inflammatory response. The overall anti-inflammatory effect of each tested extract was presented as net anti-inflammatory effect. Sample ED50 were computed with linear regression as previously reported [14].
DPPH radical scavenging assay
To examine the anti-oxidant efficacy of the extracts and of the synergy co-extract, use was made of the well-established free radical scavenging assay that employs DPPH as a radical scavenger. Prior description of the experimental method is provided [14]. Radical scavenging activity of test samples are presented in a dose-response curve that shows % inhibition versus concentration of samples. Estimation of sample EC50 was accomplished through linear regression analysis. A triplicate test for each sample concentration presents each sample point as a Mean ± SD.
Total phenolic content by Folin-Ciocalteu method
Estimation of the total phenolic contents of the extracts and of the co-extract synergy mixture was performed using the Folin-Ciocalteu reagent with protocols published elsewhere [14]. Interpolation from the standard Gallic Acid curve provided total phenolic contents of samples in Gallic Acid equivalents (GAE). A triplicate test for each sample concentration presents each sample point as a Mean ± SD.
Microbial panel
The genotypes and sources of microbial strains used in this study have previously been described [14,16]. A panel of 5 pathogenic microbial specimen that included 2 gram-positive bacteria (Streptococcus pyogenes, Staphylococcus aureus), 2 gram negative bacteria (Pseudomonas aeruginosa, Escherichia Coli) and a fungi (Candida albicans) were used as microbial targets for the assessment of extract and co-extract anti-microbial efficacy.
Earlier reports have described the genotype, storage, culture and maintenance of these microbes [16]. Working cultures of each microbe was maintained at 106 CFU/mL using McFarland standard as previously described [16].
Broth dilution assay
Extracts and synergy co-extract were tested for growth inhibitory activity against the panel of 5 pathogenic microbes using the Broth Dilution Anti-microbial Assay as previously described [14,16]. Pathogenic microbes were cultured as described [16] and split into three sets of identical cultures with each tube of cells containing 106 CFU/mL. One set was treated with Strophanthus hispidus (roots) extract while another was treated with Aframomum meleguta (seeds) extract. The third set of microbial culture received the combined Strophanthus hispidus (roots): Aframomum meleguta (seeds) co-extract treatment.
The same range of sample concentrations were utilized for pathogenic microbiocidal sensitivity: (Strophanthus hispidus (roots) extract alone (0.09 –12.5 mg/mL), Aframomum meleguta (seeds) extract alone (0.09–12.5 mg/mL) and co-extract synergy mixture maintained at 3:2 w/w ratio of Strophanthus hispidus (roots) and Aframomum meleguta (seeds) (0.09–12.5 mg/mL). Pharmaceutical drugs ciprofloxacin (anti-bacteria agent) and fluconazole (anti-fungal agent) were utilized as positive controls. Uninoculated sterile broth media with and without extracts were utilized as negative controls. Minimum Inhibitory Concentrations (MICs) were taken as the lowest extract concentration that yielded complete microbial growth inhibition.
Statistical analysis
Experiments were repeated and each sample point analyses performed in triplicates. Mean and the Standard Deviation of experimental replicates were computed with Microsoft Excel XP.
TLC
TLC demonstration that each plant extract comprise multiple chemical entities is in line with the observed varied phytochemical compositions for each extract. The three observable TLC bands for Strophanthus hispidus (roots) extract and the two discernible bands for Aframomum meleguta (seeds) extract (Table 1) are on the lower scale of chemical entities present in a crude extract and the problem might be due to the lower separatory efficiencies of the utilized chromatographic method. Nevertheless, Rf values in table 1 show that all chromatographic bands were reasonably well-resolved.
Phytochemical characterizations of extracts
Strophanthus hispidus (roots) extract is richly endowed with an array of potentially bioactive phytochemical chemotypes including saponins, flavonoids and alkaloids (Table 1). Aframomum meleguta (seeds) is also phytochemical-rich, endowed with glycosides, carotenoids and anthraquinones (Table 1). Phytochemical groups in both extracts are functionally known to evoke diverse bioactivities in chemopreventive and chemotherapeutic mechanisms including those of anti-microbial, anti-oxidant and anti-inflammatory activities. Despite the disparate plant parts (roots and seeds) and distinct plant species, Strophanthus hispidus (roots) and Aframomum meleguta (seeds) extracts showed striking details in similarities in phytochemical compositions (Table 1).
| Table 1: Thin layer chromatography (TLC) and phytochemical contents report of methanolic extracts of SH Strophanthus hispidus and of AM Aframomum melegueta plant samples. | ||
| Sample | TLC Results | Phytochemicals Present |
| Number of spots from TLC and Rf values | ||
| Strophanthus hispidus | Three 0.07, 0.19, 0.37 |
Saponins, Flavonoids, Cyanogenetic glycosides, alkaloids, Carotenoids, Anthraquinone glycosides |
| Aframomum melegueta | Two 0.14, 0.31 |
Saponins, General glycosides, Flavonoids, Cyanogenetic glycosides, Alkaloids, Carotenoids, Anthraquinones |
Ratio of individual extracts in co-extract mixture
A 3-to-2 weight/weight ratio of Strophanthus hispidus (roots) to Aframomum meleguta (seeds) derived from the best ethnomedicinal practices and guidelines was utilized for all concentrations of the co-extract in all assays (anti-inflammation, anti-microbial and anti-oxidant) as stated in Materials and Methods. Maintenance of this ratio for all co-extract concentration treatment implies that in some cases, co-extract mixtures contain reduced individual extracts concentrations relative to concentrations of either extract alone.
Anti-microbial activities
Microbial colonization of the sinus-nasal cavity plays an etiologic role in the pathophysiology of sinusitis. To assess the microbiocidal efficacy of individual Strophanthus hispidus (roots) and Aframomum meleguta (seeds) extracts and to further examine the possible synergistic anti-microbial effects of the Strophanthus hispidus (roots)-Aframomum meleguta (seeds) co-extract mixture, different doses of test samples were evaluated for relative growth inhibitory effects on a panel of 5 pathogenic microbes using Broth Dilution assay.
Bactericidal efficacies of extracts and of co-extract differed by target microorganism as shown, in quantitative terms, by the widely differing pathogen-specific range of extract MICs. The gram positive bacteria Streptococcus pyogenes showed the highest sensitivity to Strophanthus hispidus extract, succumbing to its bactericidal activities at a low MIC of 0.78 mg/mL (Tables 2- 4). The data shows no preferential display of sensitivity to the anti-proliferative effects of Strophanthus hispidus by the gram positive bacteria. Staphylococcus aureus, the other gram positive bacteria strains was the least sensitive pathogenic bacteria to Strophanthus hispidus extract’s anti-proliferative activities. Staphylococcus aureus displayed weak sensitivity with an 8-fold increase in the MIC of Streptococcus pyogenes. Strophanthus hispidus showed a reduced anti-proliferative effect on the two gram-negative bacteria strains. MICs of Strophanthus hispidus extract against the gram-negative Escherichia Coli were comparable to those recorded against the gram-negative Pseudomonas aeruginosa. Both Pseudomonas aeruginosa and Escherichia Coli showed sensitivity considered intermediate at an MIC of 3.12 mg/mL (Tables 2-4).
| Table 2: Broth dilution showing MICs for methanolic extracts of Strophanthus hispidus, Aframomum melegueta and for the co-extract mixture. | |||||||||
| Sample | Test Organisms Concentrations (mg/ml) | ||||||||
| 12.5 | 6.25 | 3.12 | 1.56 | 0.78 | 0.39 | 0.19 | 0.09 | ||
|
Strophanthus hispidus Aframomum melegueta Co-extract Mixture |
P. aeruginosa S. pyogenes E. coli S. aureus C. albicans |
- - - - - |
- - - - - |
- - - + + |
+ - + + + |
+ - + + + |
+ + + + + |
+ + + + + |
+ + + + + |
|
P. aeruginosa S. pyogenes E. coli S. aureus C. albicans |
12.5 | 6.25 | 3.12 | 1.56 | 0.78 | 0.39 | 0.19 | 0.09 | |
| - - - - + |
- - - - + |
- - - - + |
- - - - + |
- + - - + |
+ + + + + |
+ + + + + |
+ + + + + |
||
|
P. aeruginosa S. pyogenes E. coli S. aureus C. albicans |
12.5 | 6.25 | 3.12 | 1.56 | 0.78 | 0.39 | 0.19 | 0.09 | |
| - - - - - |
- - - - + |
- + - - + |
- + - - + |
- + + - + |
+ + + + + |
+ + + + + |
+ + + + + |
||
| + indicates microbial growth; - indicates no microbial growth | |||||||||
| Table 3: Broth dilution showing MICs for standard drugs (Ciprofloxacin and Fluconazole) used as positive control. | |||||||||
| Standard | Test Organisms |
Concentration (µg/ml) | |||||||
| 12.5 | 6.25 | 3.12 | 1.56 | 0.78 | 0.39 | 0.19 | 0.09 | ||
| Ciprofloxacin | P. aeruginosa | - | - | - | - | - | - | - | + |
| S. pyogens | - | - | - | - | - | - | - | + | |
| E. coli | - | - | - | - | - | - | + | + | |
| S. aureus | - | - | - | - | - | - | - | + | |
| 100 | 50 | 25 | 12.5 | 6.25 | 3.12 | 1.56 | 0.78 | ||
| Fluconazole | C. albicans | - | - | - | - | - | - | - | + |
| + indicates microbial growth; - indicates no microbial growth | |||||||||
| Table 4: Summary of MIC values of extracts and of the co-extract mixture and control drugs recorded against test organisms. | |||||
| Test Organisms | MIC (mg/ml) MIC (µg/ml) | ||||
| SH | AM | MIX | Ciprofloxacin | Fluconazole | |
| P. aeruginosa | 3.12 | 0.78 | 0.78 | 0.19 | |
| S. pyogens | 0.78 | 1.56 | 6.25 | 0.19 | |
| E. coli | 3.12 | 0.78 | 1.56 | 0.39 | |
| S. aureus | 6.25 | 0.39 | 0.78 | 0.19 | |
| C. albican | 6.25 | - | 12.5 | 0.78 | |
| SH: Strophanthus hispidus; AM: Aframomum Melegueta; MIX: Co-extract Mixture | |||||
Aframomum meleguta extract demonstrated relatively good efficacy against the two gram-negative bacteria (Pseudomonas aeruginosa and Escherichia Coli), as both yielded strongly to its antimicrobial activity at a low MIC of 0.78 mg/mL (Tables 2-4). Growth inhibitory effects of Aframomum meleguta extract on gram positives Streptococcus pyogenes and Staphylococcus aureus were also high. Aframomum meleguta extract yielded an elevated level of anti-proliferative activity with an MIC of 0.39 mg/mL on Staphylococcus aureus. Slightly lower (a 2-fold difference lower MIC) growth inhibitory activity was evoked by Aframomum meleguta against Streptococcus pyogenes (MIC of 1.56 mg/mL). Taken together, Aframomum meleguta extract was comparatively more effective in suppressing bacteria growth as it displayed a much broader range of efficacious growth inhibition against the 4 bacteria species in the panel of 5 micro-organisms.
Microbiocidal activities of the co-extract mixture against the same pathogenic microbes were mostly marked by higher MICs, or at best, by MIC values comparable to those obtained for individual extracts. For Streptococcus pyogenes, increases in MIC of the individual extracts up to 4-fold (for Aframomum meleguta alone) and up to 6-fold (for Strophanthus hispidus alone) were observed for the co-extract (Tables 2-4). MICs of co-extract required to inhibit Escherichia Coli and Staphylococcus aureus proliferation was similarly double the quantitative value of that evoked by Aframomum meleguta extract alone: MIC values for co-extract were 1.56 mg/mL for Escherichia Coli and 0.78 mg/mL for Staphylococcus aureus whereas the corresponding MIC values for Aframomum meleguta extract alone were 0.78, 0.39 mg/mL respectively (Tables 2-4). With Pseudomonas aeruginosa, anti-proliferative potencies of co-extract were comparable to those of Aframomum meleguta extract alone (MIC of 0.78 mg/mL) (Tables 2-4).
Differences in the potency of the bactericidal actions of Aframomum meleguta extract alone or of the Strophanthus hispidus extract alone or of their co-extract were observed to be significant for Staphylococcus aureus. With Staphylococcus aureus, MICs were approximately 16-fold lower with Aframomum meleguta extract than it was with Strophanthus hispidus extract. The co-extract evoked growth inhibition of Staphylococcus aureus at an approximately 2-fold lower MIC relative to that of Aframomum meleguta extract. Aframomum meleguta extract showed the strongest and broadest inhibitory activities against all bacteria with Staphylococcus aureus as the most significantly affected. With Pseudomonas aeruginosa and at MIC up to 3.12 mg/mL, Strophanthus hispidus extract showed low anti-proliferative activity. Evaluated across all three test extract samples, Pseudomonas aeruginosa can be considered the most sensitive bacteria among the panel because it succumbed to the bactericidal activities of all extract and of co-extracts at relatively low MICs (3.12 for Strophanthus hispidus; 0.78 for Aframomum meleguta and 0.78 for the co-extract mixture) (Tables 2-4).
Anti-fungal activities of extract phytochemicals were uniformly poor across the three test extract samples. Aframomum meleguta extracts did not detectably inhibit the proliferation of Candida albicans. Candida albicans was also the microorganism that was the least susceptible to the suppressive growth effects evoked by Strophanthus hispidus extract as inhibition was finally achieved at a higher MIC of 6.25 mg/mL. Co-extract fungicidal activities on Candida albicans were 2-fold difference lower than that evoked by Strophanthus hispidus extract alone (occurring at an unusually high MIC of 12.5 mg/mL) (Tables 2-4).
MICs for extract-treated microbial strains were higher (at best about 2-fold difference higher) than that of positive controls drug (Ciprofloxacin and fluconazole) indicating weaker relative anti-microbial activities of the extracts and of the co-extract mixture (Table 4).
No synergy in anti-microbial activities
The data strongly suggests that the anti-microbial efficacy of Strophanthus hispidus extract was not enhanced by the addition of Aframomum meleguta extract nor was that of Aframomum meleguta extract improved in the co-extract mixture by the added Strophanthus hispidus extract. Although microbial susceptibility to microbiocidal effects of co-extract mixtures differed according to species, attainable MICs were at best comparable to that of individual extracts. Even higher co-extract MICs were observed for some pathogenic microbes suggesting that the co-extract does not exert a synergistic anti-microbial response against this examined panel of pathogenic microbes.
Anti-Oxidant activities
Reactive oxygen species (ROS)-mediated oxidative stress trigger inflammation in pathophysiological mechanisms linked to the etiology of chronic sinusitis. To examine the anti-oxidant efficacies of individual Strophanthus hispidus (roots) and Aframomum meleguta (seeds) extracts and to additionally assess whether functional synergism exists in the anti-oxidant effects of the co-extract of Strophanthus hispidus (roots) and Aframomum meleguta (seeds), the DPPH radical scavenging assay was used.
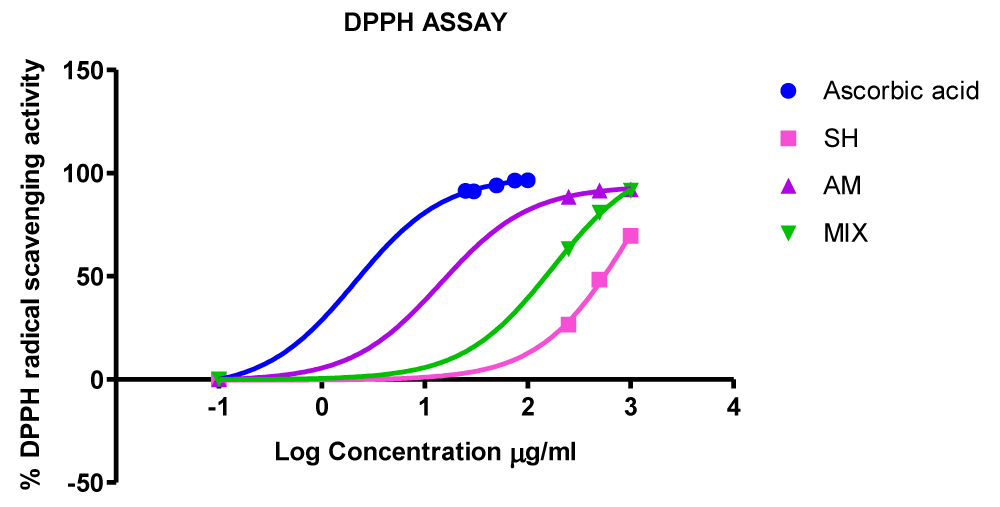
Graphical depiction of this DPPH radical scavenging efficacies highlights marginal differences in anti-oxidant activities at lower individual extract and lower co-extract concentrations (Figure 1). Differences in anti-oxidant activities, however, become well-defined at slightly higher extract concentrations where the relative scavenging responses to doses of test samples were large and where discrete quantitative DPPH scavenging values for test samples diverged. At even higher sample concentrations, DPPH scavenging activities reaches a plateau (Figure 1) and extract-dependent quantitative variations in anti-oxidant effect becomes indistinguishable.
Figure 1: Graphical depiction of the DPPH radical scavenging efficacies of the methanolic extracts of Strophanthus hispidus, Aframomum meleguta and of the co-extract mixture aligned with that of Ascorbic acid control. SH: Strophanthus hispidus; AM: Aframomum Melegueta; Co-extract Mixture(MIX).
As shown in table 5 IC50 values of test extracts were competitive with that of the Ascorbic acid control. Aframomum meleguta extract demonstrated the highest DPPH scavenging efficacy while Strophanthus hispidus extract showed the least efficacy (Figure 1 and Table 5).
| Table 5: DPPH radical scavenging activities of methanolic extracts of Strophanthus Hispidus, Aframomum meleguta and Ascorbic acid and their estimated IC50s. | |
| Sample | IC50 µg/ml |
| Ascorbic acid | 2.093 |
| SH | 969.1 |
| AM | 14.30 |
| MIX | 173.5 |
| SH: Strophanthus hispidus; AM: Aframomum Melegueta; MIX: Co-extract Mixture | |
Although the co-extract was a strong anti-oxidant with an IC50 of 173.5 µg/mL, it still lagged in efficacy relative to Aframomum meleguta (seeds) extract as judged by relative IC50 values (Table 5) and as further assessed by the graphical depiction of dose-response values in figure 1. Strophanthus hispidus extract (IC50 = 969.1 µg/mL), on the other hand is a 5-fold difference less potent DPPH radical scavenger relative to the co-extract mixture. Quantitatively, the IC50 value of the co-extract is sandwiched between that of the two individual Strophanthus hispidus and Aframomum meleguta extracts and its relative median position effectively mirrors the trend in Total Phenolic Content (Tables 5,6).
| Table 6: Total Phenolic Content of Strophanthus hispidus and Aframomum meleguta (gGAE/100g). | |
| Sample | Phenolic Content (gGAE/100g) |
| SH | 20.4 |
| AM | 57.71 |
| MIX | 38.57 |
| SH: Strophanthus hispidus; AM: Aframomum Melegueta; MIX: Co-extract Mixture | |
Total phenolic content of Aframomum meleguta seed extracts (57.71 mg GAE/100 g) were about 3-fold difference higher than that of Strophanthus hispidus root extracts. The co-extract mixture, nominally, showed higher phenolic content than Strophanthus hispidus extract alone but displayed a lower phenolic content than Aframomum meleguta extract alone. Taken together, the increasing trend in total phenolic contents of test extracts largely mirrors the increasing quantitative trends in observed DPPH scavenging activities.
No synergy in anti-oxidant activities
Viewed through the lens of phytochemical interactions within the co-extract mixtures, Strophanthus hispidus extract depressed the scavenging efficacy of Aframomum meleguta extract phytochemicals in the co-extract mixture as its IC50 in the individual extract reduced by 12-fold when mixed in the co-extract. On the other hand, the addition boosted the scavenging activities of Strophanthus hispidus extract as the overall IC50 of the co-extract mixture increased by about a 5-fold difference when compared to that of the Strophanthus hispidus extract alone. Co-extract mixtures evoked radical scavenging activity that are comparable to that of the individual extracts. Consequently, when assessed via IC50s the co-extract mixture did not induce synergistic scavenging of DPPH radicals in its in vitroi anti-oxidant response.
Anti-inflammation activities
Inflammation of the sinus-nasal mucosa is a hallmark etiologic feature of chronic sinusitis. To examine whether individual extracts and the co-extract mixture inhibit inflammation, samples were assessed for their relative efficacies to reduce the swelling induced by carrageenan treatment of the foot of 7-day old chicks. Measured inhibition in oedematous foot volume triggered by individual extracts and by the co-extract mixture was taken as the direct quantitative estimate of the anti-inflammatory effect of plant phytochemicals as described in Material and Methods. Anti-inflammatory responses of test samples were quantitatively presented as a time-course event (Figures 2,3) and further evaluated as a dose response outcome (Figures 4,5).
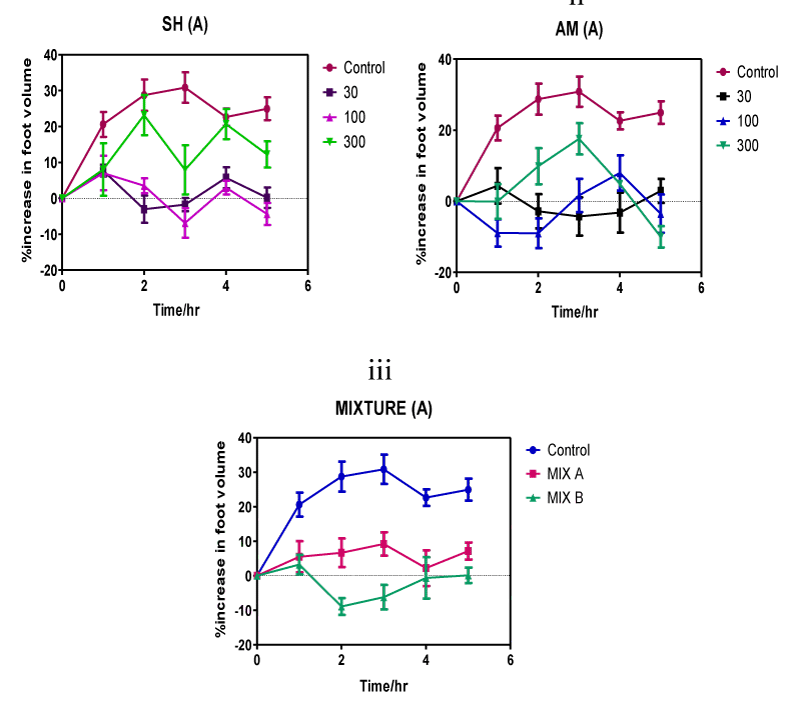
Figure 2: Time course of oedema volume changes in the carrageenan-induced inflammation of the 7-day chick foot triggered by individual Strophanthus hispidus (SH) extract (i) and Aframomum melegueta (AM) extract (ii) and by the Strophanthus hispidus-Aframomum melegueta co-extract mixture (MIX) (iii). Co-extract induced inhibition in oedematous volume utilizes two different doses-3 mg/kg (MIX A) and 10 mg/kg (MIX B) of the mixture of extracts.
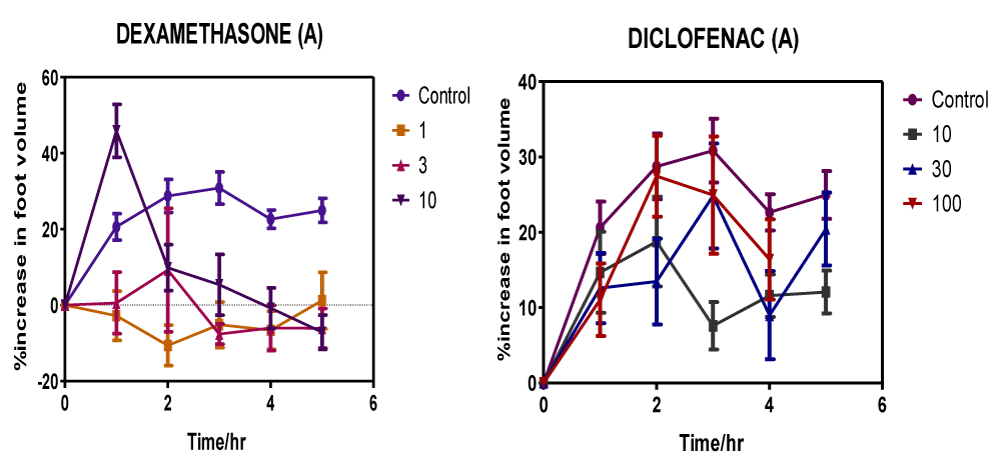
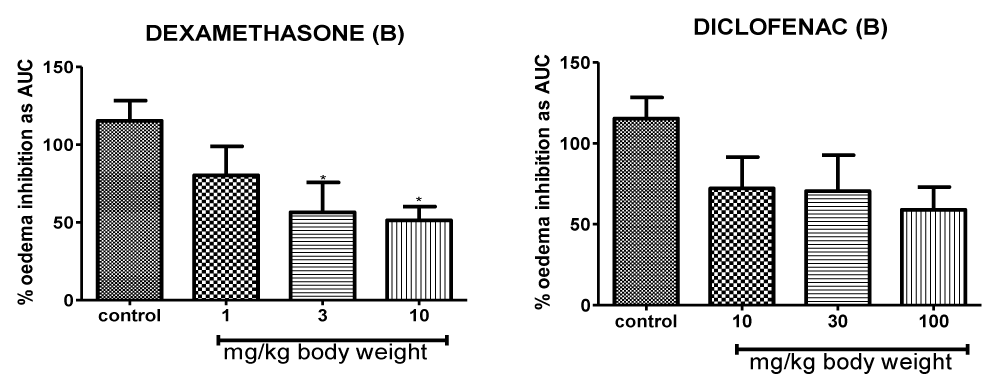
Figure 3: Time course measurements of the anti-inflammatory response of Dexamethasone and Diclofenac on carrageenan-induced foot oedema in 7-day chicks.
Figure 4: Dose-response of anti-inflammatory activity evoked by individual Strophanthus hispidus (SH) extract (i) and Aframomum melegueta (AM) extract (ii) and by the Strophanthus hispidus-Aframomum melegueta co-extract mixture (MIX) (iii) on the carrageenan-induced inflammation of the 7-day chick foot. Co-extract induced inhibition in oedematous volume utilizes two different doses-3 mg/kg (Mix A) and 10 mg/kg (Mix B) of the mixture of extracts. All test samples (extracts and co-extract,) reduced oedema volumes in a dose-dependent manner in the chick-feet carrageenan oedema assay. ***P < 0.001, **P < 0.01 and *P < 0.05 compared to the saline-treated group.
Figure 5: Inflammation suppressive efficacy on the carrageenan-induced oedema of the 7-day chick foot triggered by positive control drugs Dexamethasone and Diclofenac. Control drugs reduced oedema volumes in a dose-dependent manner.
Time-course of inflammation inhibition
Anti-inflammatory responses of individual extracts and of the co-extract follows a graded continuum of efficacy that varies with increasing post treatment (p.t.) time. Suppressive effects of 300 mg/kg dose of Strophanthus hispidus (roots) extract on carrageenan-induced swelling of chick feet commences within 1 h after treatment (Figure 2(i)) and reaches a peak of 35% inhibition after 3 h before it recedes in magnitude to 10% after 5 h. The lower dose of 100 mg/kg Strophanthus hispidus (roots) extract showed reduced effectiveness against the swelling exhibiting a maximal 25% reduction after 2 h and a 5% after 3 h before ending with 1% at the eventual completion of the experiment at 5 h. The lowest dose (30 mg/kg) of Strophanthus hispidus (roots) extract evoked a marginal anti-inflammatory response that peaked at 15%, 2 h after ingestion. Beyond 2 h, anti-inflammatory response per unit time for the 30 mg/kg dose decreased significantly until completion of the experiment at 5 h.
Treatment with Aframomum meleguta (seeds) extract reveals a time-course of inflammation inhibition qualitatively identical to that of Strophanthus hispidus (roots) (Figure 2(ii)). Anti-inflammation response initiated by the 300 mg/kg dose of Aframomum meleguta (seeds) extract starts 1 h after extract treatment and reaches a peak anti-inflammatory response of 15% after 5 h. Chicken treated with the intermediate dose of Aframomum meleguta (seeds) extracts (100 mg/kg) reached a maximum mean reduction of swelling of 15% after 1 h. After 2 h of treatment, 30 mg/kg dose of Aframomum meleguta (seeds) extract suppressed carragenan-induced inflammation by 5%. The inhibitory effects of this lowest dose (30 mg/kg) of Aframomum meleguta (seeds) extracts peaked after 3 h but was the lowest recorded of the series of maximal inhibitions (5%) and it further declined in intensity to 1% after 5 h.
Time course of anti-inflammatory response evoked by the Strophanthus hispidus (roots)-Aframomum meleguta (seeds) co-extract showed brief trends of increasing anti-inflammatory effects within the 5 h timeline (Figure 2(iii)). Chicken treated with the lower dose of co-extract (3 mg/kg) experienced reduced swelling of the foot to below 10% after 4 h p.t. and to less than 5% at the completion of experiment 5 h p.t. Maximum inhibitory effects triggered by this 3 mg/kg dose therefore occurred 4 h after co-extract treatment. Inflammation inhibition intensifies at the higher dose (10 mg/kg) of co-extract showing a co-extract time-course that has a highest rate of inflammation suppression of 10% (2 h p.t.), 7% (3 h p.t.) and 2% (4 h p.t.). Peak inhibition induced by the 10 mg/kg dose of the co-extract mixture occurred at a considerably shorter time (2 h) and was fairly sustained for a longer duration (approximately 2 h) than for individual extract.
Inflammation suppressive effects for control drugs across the different dose ranges (1-10 mg/kg for Dexamethasone; 10-100 mg/kg for Diclofenac) within the 0-5 h time course range reveals relatively higher anti-inflammatory efficacies compared to that of test extracts (Figure 3). The lowest dose (1 mg/kg) of the steroidal anti-inflammatory drug dexamethasone demonstrated significant inhibition of inflammation compared to vehicle-treated controls as it triggered a 15% maximal reduction in oedema volumes after 2 h (Figure 3). A 3 mg/kg dose of dexamethasone lowered the inflammatory response by 10% in the foot oedema at 3 h p.t. A surprisingly weaker anti-oedematous response occurred at the highest dose of 10 mg/kg where the drug-induced decrease in inflammation was a marginal 1% after 4 h.
After 3 h p.t. the 10 mg/kg dose of diclofenac - the structurally unrelated nonsteroidal anti-inflammatory agent evoked a maximum anti-inflammatory response of 5%. Inflammation decreased by approximately 4% in response to 30 mg/kg dose of diclofenac (Figure 3) at 4 h p. t. and by about 2% in response to 100 mg/kg dose at 1 h p. t. Maximum anti-inflammatory response for diclofenac was recorded by the 10 mg/kg dose as 5% at 3 h p. t.
Dose-response of test samples in anti-inflammation activities
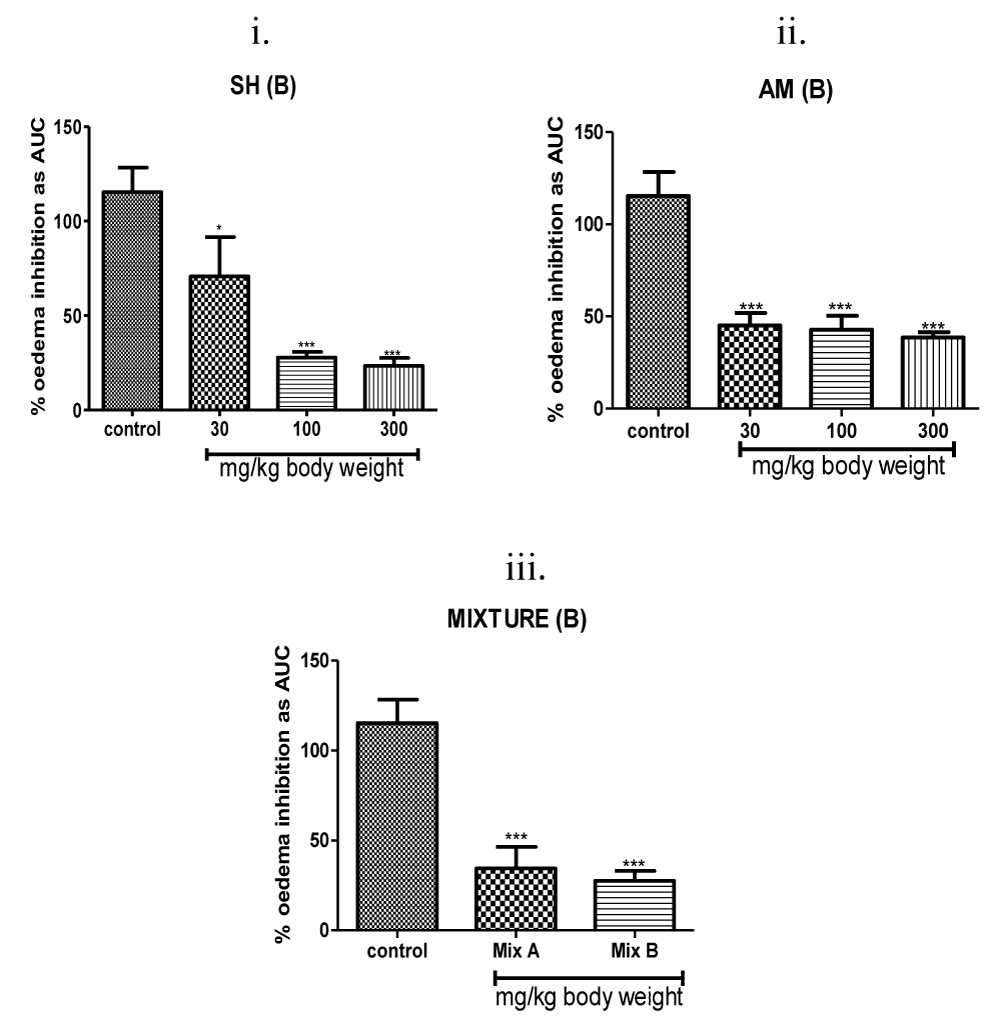
Graded anti-inflammatory responses evoked by the 7-day old chicks following ingestion of graded doses of test samples in the carrageenan-induced chick feet assay are shown in (Figure 4). In all test cases, average inflammation inhibitory responses correlated quantitatively with graded doses of administered test samples including those of the individual extracts, co-extract and control drugs (p < 0.05).
A 300 mg of Strophanthus hispidus (roots) extract yielded an approximate 80% inhibition of inflammation while a lower dose of 100 mg yielded 75% anti-oedematous effect. Chicken treated with a lowest dose of Strophanthus hispidus (roots) extracts (30 mg/kg) had mean reduction of swelling of 30% (Figure 4(i)). A quantitative and qualitative pattern of dose–response relationship comparable to that of Strophanthus hispidus (roots) was observed with that of Aframomum meleguta (seeds) where the 300 mg/kg of its extract gave 70% anti-inflammatory response (Figure 4(ii)). A 62% suppressive effect was obtained after 100 mg/kg dose of Aframomum meleguta (seeds) extract treatment. Feeding the chicks with 30 mg/kg of Aframomum meleguta (seeds) extract evoked up to 60% inhibition of inflammation.
Similar to the anti-inflammation triggered by the individual extracts, a display of a clear correlation between the concentration of the Strophanthus hispidus (roots)-Aframomum meleguta (seeds) co-extract mixture and the suppressive volume of oedema levels was noted (Figure 4(iii)). At 10 mg/kg, co-extract reduced the swelling of feet by 80% (Figure 4(iii)). A lower dose of co-extract (3 mg/kg) proportionally reduced swelling by 75% (Figure 4(iii)). Co-extract suppressive efficacies of the two tested lower doses (3, 10 mg/kg i.p.) were quantitatively comparable to the oedema suppression yielded by the highest doses (300 mg/kg) of individual Strophanthus hispidus (roots) and Aframomum meleguta (seeds) extracts producing at least 10-fold dose difference. In fact co-extract treatment of 3 mg/kg was a more effective anti-inflammatory agent than the higher dose (100 mg/kg) of Strophanthus hispidus (roots) extract alone or of Aframomum meleguta (seeds) extract alone.
Comparatively lower doses of diclofenac (10, 30, and 100 mg/kg i.p) were far more effective anti-inflammatory agents than the higher doses of the individual extracts (30, 100 and 300 mg/kg i.p) yielding, respectively 30%, 33% and 45% reduction in the swelling of feet (Figure 5). Anti-inflammatory efficacies higher than that of diclofenac were observed in response to graded quantitatively lower doses of dexamethasone (1, 3, and 10 mg/kg i.p). Dexamethasone treatment (1.0, 3.0, 10.0 mg/kg, i.p) was similarly more effective than the higher doses (30, 100 and 300 mg/kg i.p) of both individual Strophanthus hispidus (roots) extract and separate Aframomum meleguta (seeds) extracts yielding respectively 30%, 45% and 55% suppressive effects.
Synergy in anti-inflammation activities
The data shows that co-extract mixture evoked anti-inflammatory potency on a quantitative scale comparable to that shown by the two control drug. Co-extract at 3 mg/kg significantly reduced the mean feet oedema of the chicks to levels comparable to that of the steroidal positive control drug dexamethasone at 1 mg/kg and to the nonsteroidal anti-inflammatory drug Diclofenac at 30 mg/kg (Figures 4,5). Also, the anti-inflammatory effect of the co-extract at 10 mg/kg represents at least a 3-fold greater suppressive effect relative to 100 mg/kg of Strophanthus hispidus (roots) extract and a 2-fold higher inhibitory effect relative to 100 mg/kg of Aframomum meleguta (seeds) extract (Figure 4). Co-extracts was similarly robust at lower concentrations as its co-administration at 3 mg/kg yielded at least a 3-fold higher anti-inflammation in the chick foot oedema relative to either Strophanthus hispidus (roots) or Aframomum meleguta (seeds) extracts alone at 10 mg/kg (Figure 4). In fact, the combined effects of Strophanthus hispidus (roots) extract and Aframomum meleguta (seeds) extract were synergistic (an overall 4–7-fold greater anti-inflammatory effect).
Comparative EC50 values of test extracts in anti-inflammation activities
Relative EC50 values of test samples were a consistent match with observed dose-responses of anti-inflammatory effects. The Strophanthus hispidus (roots)-Aframomum meleguta (seeds) co-extract exhibited a highest potency of the three test extracts with an EC50 value comparable to that of the standard control drugs dexamethasone and diclofenac (Table 7). With an EC50 of 117 (4-fold less potent than that of the co-extract), Strophanthus hispidus (roots) extract was a fourth as potent as the co-extract mixture. Aframomum meleguta (seeds) extract showed the lowest potency in the series of tested extracts with an EC50 that is 7-fold higher than that of the co-extract mixture. Estimated EC50 values of test extracts followed the order: Strophanthus hispidus (roots)-Aframomum meleguta (seeds) co-extract mixture < Strophanthus hispidus (roots) extract < Aframomum meleguta (seeds) extract.
Key observations derived from this study were that crude co-extract phytochemical pool originated from the mixture of Strophanthus hispidus (roots) and Aframomum meleguta (seeds) extracts synergistically inhibits inflammation in vivo and nominally suppresses microbial growth and scavenges free radicals in vitroi. Collective bioactivities were triggered by multi-constituent and phytochemically-diverse extracts. And their evocation is unsurprising because such multifunctional phytochemical groups including saponins, flavonoids, alkaloids, glycosides, carotenoids and anthraquinones are broadly known to provide protective effects against inflammation, to offer effective microbiocidal activities and to enhance the ability of cells to scavenge ROS and to resist oxidative stress [7-13].
The diversity of phytochemical compositions affirms, in mechanistic terms, the execution of multi-mechanisms at multi-target sites by bioactive constituents [17]. The variety of chemotypes of phytochemicals, the miscellany of phytochemical targets, the multiplicity of phytochemical-dependent mechanistic action and the assortment of mechanistic inter-relationships evoked by different extract phytochemicals within the pool of bioactive constituents likely account for the varied scope of observed bioactivities. Differences in phytochemical chemotypes as well as variability in their relative quantitative amounts account for differences in the relative extract potencies exhibited by extracts and co-extracts for the three examined bioactivities.
Within this context, the observed synergy in the co-extract anti-inflammation is likely attributable to its wider chemodiversity pool that enables multiple and complementary phytochemicals access to new target sites inaccessible or unavailable to the limited pool of phytochemicals in the individual extracts [18]. Possibly, pooled phytochemicals from both extracts within the co-extract mixture might functionally cooperate as their collective activities overlap and reinforce each other into a net more potent complementary whole [4,5]. Literature reports attribute the swelling of chick foot in the carrageenan-induced inflammations to dysregulated NF-kB pathway genetic activities [19]. The data suggests that phytochemical combinations from the co-extract complementarily induce and/or inactivate NF-kB pathway genes to potentiate a more effective anti-inflammatory response. Dependence of anti-inflammation effect on extract concentration shows that functional mechanistic engagement of phytochemicals on the NF-κB pathway gene is calibrated physiologically to correspond with graded doses of extracts.
Functional linkage between the key molecular participants involved with the co-extract phytochemical responses to the anti-inflammation, anti-microbial and anti-oxidant activities was not established. Extract phytochemicals act as anti-oxidants presumably through the induction of genes that are involved in the maintenance of cellular redox balance with activities that include de-activation of pathways that generate reactive oxygen species (ROS) or triggering pathways that increases cellular resistance to oxidative stress in vivo [20].
Although the co-extract emerges as moderately inhibitory to ROS generation, it nevertheless falls short of the scavenging activity evoked by Aframomum meleguta extract. The lack of increase in DPPH scavenging efficacy of the co-extract over that of the Aframomum meleguta extracts implies that the customary increases in the diversity of phytochemical chemotypes afforded by the co-extracts did not lead to a more active inhibitory effect on ROS generation or more broadly, did not offer a more potent anti-oxidant effect. Co-extract phytochemical constituents triggered either an up-regulation and/or down-regulation of some network genes whose gene products allow the cellular machinery to trigger just a nominal anti-oxidant response. As with anti-inflammation, extracts and co-extracts dose-dependently scavenged DPPH radicals indicating the reliance of DPPH scavenging on the quantitative amount of the co-extract’s polyphenolic and other anti-oxidant phytochemical content.
The demonstration of a broad spectrum microbiocidal effect by the co-extract is in line with its observed phytochemical diversity. Despite the lack of consistency in maintaining the lowest MIC with some microbes, the weight of the overall experimental evidence points to the co-extract as the modestly potent microbicide. However the underlying reason for the failure of the co-extract to evoke synergy in its microbiocidal effect can only be speculated. Mechanistically, extract phytochemicals likely target multiple anti-microbial molecular events such as efflux pump inhibitory signaling and quorum quenching signaling pathways leading to the eventual exhibition of bactericidal and/or bacteriostatic action of the extracts and of the co-extracts [21]. The lack of any synergistic microbiocidal effect on pathogenic microorganisms suggests that none of the operative mechanism of inhibition utilized by individual phytochemicals in distinct extracts complements or reinforces the other when combined in the co-extract. The possibility also exist that there might be mutually antagonistic activities of individual phytochemicals in the pool as their activities at specific sites might oppose activities at other sites. Similar anti-oxidant and anti-microbial patterns of efficacy of individual plant extracts have been confirmed [7-13].
Co-extract phytochemicals likely target multiple genetic molecular events such as impairment of redox balance, anti-microbial and anti-inflammatory signaling involved in sinusitis. These bioactive effects of the extracts are possibly inter-related and they afford as biochemical consequence prevention, growth control and therapy of sinusitis. Thus, the observed synergy in anti-inflammatory action of the co-extracts probably reflects not just activation/deactivation of NF-kB genes but also effects on many other important mediators of inflammation that are at the intersection of anti-oxidant and anti-microbial activities. Past reports on phytochemical synergy are instructive for its use of at least one pure compounds as one of the synergists [22-29]. And scientifically supported examples of phytochemical synergy in the ethnomedicinal remedy terrain are not rare [4,5,30].
Deep historical knowledge about its lack of toxicity and enduring experiential knowledge about its good efficacy against chronic sinusitis continue to support the clinical utility of the co-extract in ethnomedicine. Despite the wealth of scientific information on individual uses, there is surprisingly no literature report that has defined the acute and chronic toxicity profiles of both plants. Such toxicity concerns will ultimately have to be addressed scientifically if widespread clinical utility across both ethnomedicinal and allopathic medicine is desired. The identification of phytochemical synergy in its anti-inflammatory action is of high clinical significance and its importance goes beyond the obvious offer of therapeutic advantage over use of individual plants. Potential structurally and mechanistically diverse phytochemical set from the co-extract mixture may enhance chronic sinusitis management outcomes or may even help to achieve durable clinical control of the disease. Altogether, the clinical significance of the study is that Strophanthus hispidus (roots) extract in combination with Aframomum meleguta (seeds) extract provides a superior therapeutic index through mechanistic synergy that offers health beneficial advantage in the ethnomedicinal management of chronic sinusitis.
Between the phytochemical synergism in anti-inflammation effects on one hand and non-synergistic, non-additive anti-oxidant and anti-microbial effects on the other, the mechanistic interpretation of the bioactivities of the co-extract is currently mired in speculation. But how the co-extract phytochemicals trigger these divergent biochemical effects are issues of current interest. The discovery of the identity of the phytochemical synergistic, the detection of the putative molecular targets and the assessment of the mechanistic inter-relationship between the phytochemical synergists for its potent anti-inflammatory response will move this study towards the much needed phytochemical mechanistic specificity.
In Ghanaian ethnomedicine where the mixture of aqueous extracts of Strophanthus hispidus (roots) and Aframomum meleguta (seeds) is known to possess significant chemotherapeutic effects against chronic sinusitis, no evidentiary scientific support base for this enduring expertise exist. This study shows that potential phytochemical synergy between extracts of Strophanthus hispidus (roots) and Aframomum meleguta (seeds) evokes a robust anti-inflammatory activity in vivo. Methanol extract of each plant shows high phytochemical diversity with functionalities including flavonoids, alkaloids and anthraquinones that have frequently been implicated in multiple bioactivities such as those of anti-microbial, anti-oxidant and anti-inflammation. Overall, individual extracts and co-extracts showed a broad range of microbicide efficacies across a panel of 5 pathogenic microbes. Individual extracts demonstrated stronger anti-microbial efficacies against the microbial test panel than did the co-extracts mixture. Co-extract mixtures and individual extracts showed comparable potencies in in vitroi anti-oxidant DPPH radical scavenging assays.
Co-extract-mediated suppression of carrageenan-induced swelling of chick foot exceeded the quantitative sum of that of the individual-extract-mediated anti-inflammatory responses. Taken together, these observations are strongly suggestive of a lack of phytochemical synergies or even of the absence of functional mechanistic additiveness for anti-microbial and anti-oxidant properties but are indicative of a synergistic mode of functionality of phytochemicals in the co-extract for anti-inflammation. This study concludes that the use of the co-extracts of Strophanthus hispidus (roots) and Aframomum meleguta (seeds) will significantly inhibit microbial activities, will provide substantial antioxidant effects and will exert potent synergistic anti- inflammatory efficacy. Optimal chemopreventive and chemotherapeutic potential of both plants as anti-inflammatory agent therefore resides in the co-extract where its pool of bioactive phytochemicals functionally interact to generate synergy. The study therefore lays the groundwork for a larger future study whose focus will be on the identification of the specific phytochemicals responsible for the synergy in the anti-inflammatory response. Isolation of the synergistic anti-inflammatory phytoconstituent(s) could propel Strophanthus hispidus (roots) and Aframomum meleguta (seeds) ethnomedicinal recipe in a new direction towards drug discovery.
- Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001; 8: 401-409. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/11695885
- Komape NP, Bagla VP, Kabongo-Kayoka P, Masoko P. Anti-mycobacteria potential and synergistic effects of combined crude extracts of selected medicinal plants used by Bapedi traditional healers to treat tuberculosis related symptoms in Limpopo Province, South Africa. BMC Complement Altern Med. 2017; 17: 128-135. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28235402
- Liu Z, Luo Z, Jia C, Wang D, Li D. Synergistic Effects of Potentilla fruticosa L. Leaves Combined with Green Tea Polyphenols in a Variety of Oxidation Systems. J Food Sci. 2016; 81: 1091-1101. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27061936
- Ahounou JF, Ouedraogo GG, Gbenou JD, Ouedraogo S, Agbodjogbe WK, et al. Spasmolytic effects of aqueous extract of mixture from Aframomumum melegueta (K Schum) - Citrus aurantifolia (Christm and Panzer) on isolated trachea from rat. Afr J Tradit Complement Altern Med. 2011; 9: 228-233. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23983339
- Brahmbhatt M, Gundala SR, Asif G, Shamsi SA, Aneja R. Ginger phytochemicals exhibit synergy to inhibit prostate cancer cell proliferation. Nutr Cancer. 2013; 65: 263-272. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23441614
- Wallig MA, Heinz-Taheny KM, Epps DL, Gossman T. Synergy among phytochemicals within crucifers: does it translate into chemoprotection? J Nutr. 2005; 135: 2972S-2977S. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16317157
- Fageyinbo MS, Akindele AJ, Adenekan SO, Agbaje EO. Evaluation of in-vitro and in-vivo antidiabetic, antilipidemic and antioxidant potentials of aqueous root extract of Strophanthus hispidus DC (Apocynaceae). J Complement Integr Med. 2019; 16. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31318693
- Ishola IO, Awodele O, Oreagba IA, Murtala AA, Chijioke MC. Antinociceptive, anti-inflammatory and antiulcerogenic activities of ethanol root extract of Strophanthus hispidus DC (Apocynaceae). J Basic Clin Physiol Pharmacol. 2013; 24: 277-286. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23729560
- Agyare C, Dwobeng AS, Agyepong N, Boakye YD, Mensah KB, et al. Antimicrobial, Antioxidant, and Wound Healing Properties of Kigelia africana (Lam.) Beneth. and Strophanthus hispidus DC. Adv Pharmacol Sci. 2013; 2013: 692613. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23662099
- Freiesleben SH, Soelberg J, Jäger AK. Medicinal plants used as excipients in the history in Ghanaian herbal medicine. J Ethnopharmacol. 2015; 174: 561-568. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25773489
- Ilic NM, Dey M, Poulev AA, Logendra S, Kuhn PE, et al. Anti-inflammatory activity of grains of paradise (Aframomum melegueta Schum) extract. J Agric Food Chem. 2014; 62: 10452-10457. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25293633
- Onoja SO, Omeh YN, Ezeja MI, Chukwu MN. Evaluation of the in vitroi and in vivo Antioxidant Potentials of Aframomum melegueta Methanolic Seed Extract. J Trop Med. 2014; 2014: 159343. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24955096
- Abdel-Naim AB, Alghamdi AA, Algandaby MM, Al-Abbasi FA, Al-Abd AM, et al. Phenolics Isolated from Aframomum meleguta Enhance Proliferation and Ossification Markers in Bone Cells. Molecules. 2017; 22. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28869564
- Mensah JK, Amarh MA. Antioxidant, antimicrobial and anti-inflammation activities of Telfairia occidentalis seeds extract. Current Science Perspectives. 2019; 5: 14-23.
- Trease GE, and Evans WC. Ed. Pharmacology, 12th Edn. Bailliere Tindal Macmillan Publishers: London UK.1984; 257.
- Mensah JK, Golomeke D. Antioxidant and antimicrobial activities of the extracts of the Calyx of Hibiscus Sabdariffa Linn. Current Science Perspectives 2015; 1: 69-76.
- Caesar LK, Cech NB. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat Prod Rep. 2019; 36: 869-888. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31187844
- Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004; 134: 3479S-3485S. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15570057
- Borthakur A, Bhattacharyya S, Anbazhagan AN, Kumar A, Dudeja, PK, et al. Prolongation of carrageenan-induced inflammation in human colonic epithelial cells by activation of an NFκB-BCL10 loop. Biochim Biophys Acta. 2012; 1822: 1300–1307. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22579587
- Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, et al. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018; 413: 122-134. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29113871
- Perumal Samy R, Gopalakrishnakone P. Therapeutic Potential of Plants as Anti-microbials for Drug Discovery. Evid Based Complement Alternat Med. 2010; 7: 283-294. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18955349
- Lin YT, Labbe RG, Shetty K. Inhibition of Listeria monocytogenes in fish and meat systems by use of oregano and cranberry phytochemical synergies. Appl Environ Microbiol. 2004; 70: 5672-5678. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15345457
- Lin YT, Kwon YI, Labbe RG, Shetty K. Inhibition of Helicobacter pylori and associated urease by oregano and cranberry phytochemical synergies. Appl Environ Microbiol. 2005; 71: 8558-8564. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16332847
- Hsieh TC, Wu JM. Suppression of cell proliferation and gene expression by combinatorial synergy of EGCG, resveratrol and gamma-tocotrienol in estrogen receptor-positive MCF-7 breast cancer cells. Int J Oncol. 2008; 33: 851-859. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/18813800
- Vattem DA, Jang HD, Levin R, Shetty K. Synergism Of Cranberry Phenolics With Ellagic Acid And Rosmarinic Acid For Antimutagenic And DNA Protection Functions. J Food Biochemistry. 2006: 30: 98-116.
- Malongane F, McGaw LJ, Mudau FN. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: a review. J Sci Food Agric. 2017; 97: 4679-4689. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28585285
- Zhou JR, Yu L, Zhong Y, Blackburn GL. Soy phytochemicals and tea bioactive components synergistically inhibit androgen-sensitive human prostate tumors in mice. J Nutr. 2003; 133: 516-521. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/12566493
- Ayaz M, Ullah F, Sadiq A, Ullah F, Ovais M, et al. Synergistic interactions of phytochemicals with antimicrobial agents: Potential strategy to counteract drug resistance. Chem Biol Interact. 2019; 308: 294-303. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31158333
- Essid R, Hammami M, Gharbi D, Karkouch I, Hamouda TB, et al. Antifungal mechanism of the combination of Cinnamomum verum and Pelargonium graveolens essential oils with fluconazole against pathogenic Candida strains. Appl Microbiol Biotechnol. 2017; 101: 6993-7006. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28766033
- Zhang L, Virgous C, Si H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J Nutr Biochem. 2019; 69: 19-30. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31048206