More Information
Submitted: January 23, 2024 | Approved: February 12, 2024 | Published: February 13, 2024
How to cite this article: Mohammed Z, Muskan MZ, Narayanan MM. Acyclovir Induced Acute Kidney Injury: A Case Report. Arch Pharm Pharma Sci. 2024; 8: 001-002.
DOI: 10.29328/journal.apps.1001048
Copyright License: © 2024 Mohammed Z, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and repro-duction in any medium, provided the original work is properly cited.
Keywords: Acyclovir; Acute kidney injury; Serum creatinine
Acyclovir Induced Acute Kidney Injury: A Case Report
Ziauddin Mohammed1*, Mariya Zoha Muskan2 and Megha Mohan Narayanan2
1Department of Clinical Pharmacology, Apollo Hospitals, Jubilee Hills, Hyderabad, Telangana, India
2Department of Pharmacy Practice, Malla Reddy Pharmacy College, Hyderabad, Telangana, India
*Address for Correspondence: Dr. Ziauddin Mohammed, Department of Clinical Pharmacology, Apollo Hospitals, Jubilee Hills, Hyderabad, Telangana, India, Email: [email protected]
Herpes zoster ophthalmicus, commonly referred to as shingles, manifests as a painful skin rash affecting one or more dermatome distributions of the trigeminal nerve, which supplies sensory innervation to the eye and its surrounding structures. Acyclovir stands as the primary pharmacological intervention for the treatment of this condition. However, its administration is associated with a notable risk of adverse effects, with acute kidney injury being the most prevalent. Herein, we present a case report involving a 59-year-old female patient who developed acute kidney injury after the prescription of Acyclovir for the management of herpes zoster ophthalmicus. This case underscores the importance of vigilance regarding potential renal complications associated with Acyclovir therapy, particularly in susceptible patient populations.
A viral disease known as Herpes Zoster ophthalmicus causes a painful skin rash in one or more dermatome distributions of the fifth cranial nerve (trigeminal nerve), which runs through the eye and ocular adnexa. The primary medication in the treatment of Herpes Zoster ophthalmicus is Acyclovir. Acyclovir is utilized for alleviating symptoms associated with conditions such as chickenpox, shingles, herpes virus infections affecting the genitals, skin, brain, and mucous membranes like the lips and mouth. Additionally, it is employed in treating widespread herpes virus infections in newborns and as a preventative measure against recurrent genital herpes infections [1]. It frequently causes crystal nephropathy, which is a common mechanism for the adverse effect. By functioning as an analogue of deoxyguanosine triphosphate, acyclovir inhibits viral DNA polymerase in a competitive manner [2]. The medication enters the bloodstream and travels to the renal tubules, where it crystallizes and can cause kidney damage. When a kidney injury occurs, the serum creatinine level rises rapidly. Usually, the side effect is treated by stopping the medication, and urine output should be normal. A decrease in renal function that typically occurs 12 to 48 hours after the administration of acyclovir, as indicated by an increase in serum creatinine, is an indication of acute kidney injury, which is the result of the administration of acyclovir. When compared to oral administration, parenteral administration speeds up the drug’s absorption into the bloodstream and is associated with a higher incidence of side effects, according to studies [3]. Acyclovir intravenous infusion is typically linked to the side effects of acute kidney injury. Acyclovir falls under the category of medications known as antivirals, employed in addressing infections triggered by viruses.
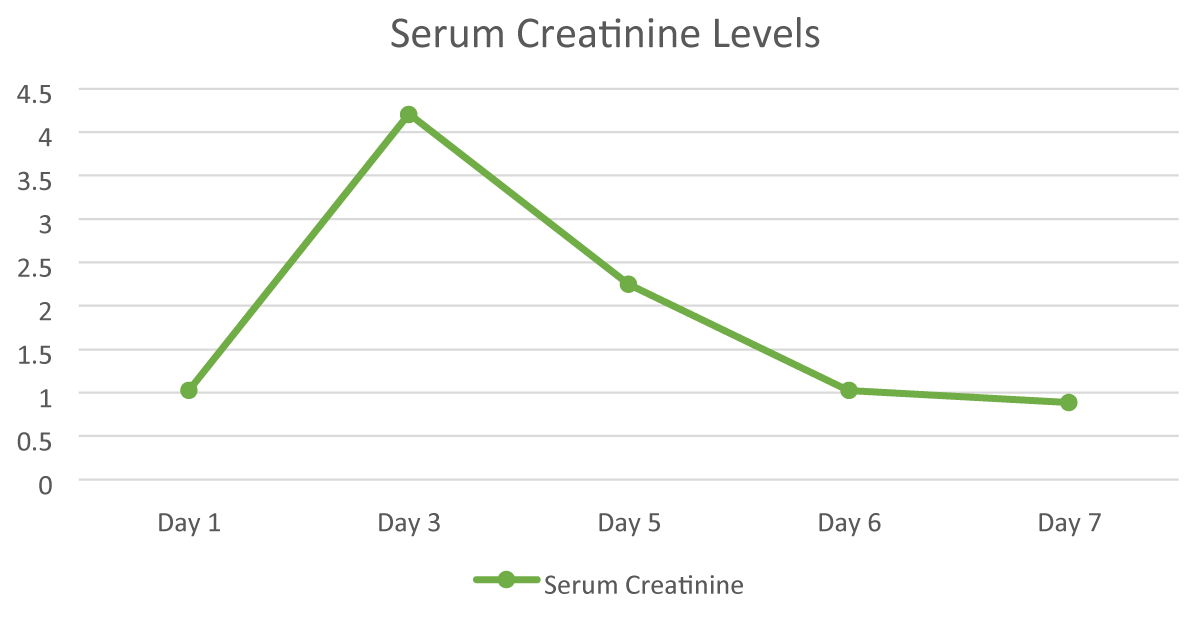
A 59-year-old female presented with complaints of left-sided headache and facial pain, lowgrade fever associated with vesicular lesions over the left cheek and left palate for 1 week followed by decreased vision, redness over the face, and watering over the left eye associated with vomiting and was brought to the hospital for further management. Past Medical History included Hypertension for 3 years (on T. Nifedipine 20 mg, once a day). The patient was diagnosed with herpes zoster ophthalmicus and she was started on Acyclovir and neuropathic pain medications for headache. The routine laboratory reports were within the normal range such as the Complete Blood Count (CBC) and Liver Function Test (LFT), Erythrocyte Sedimentation Rate (ESR) was 31 mm/hr, and Serum creatinine was 1.03 mg/dl. The Cockcroftgault method was used to determine the creatinine of the patient. The patient developed cerebellar signs (horizontal nystagmus fast pointing towards left and left finger nose test was positive which showed upper limb ataxia). An ophthalmology opinion was taken, and the advice was followed. Suspecting viral cerebellitis, MRI Brain with contrast was done and left mastoiditis was diagnosed with no acute changes. As the MRI was inconclusive, a lumbar puncture was done. CSF analysis indicated lymphocyte predominant, high protein, normal glucose meningitis, varicella zoster PCR was positive, and routine cultures showed no growth. The patient’s serum creatinine level was 1.03 mg/dl on the day of admission. Acyclovir 750 mg IV three times a day was administered to treat the zoster virus, the serum creatinine level was increased up to 4.21 mg/dl by the third day of admission after 8 doses which indicated nonoliguric Acute Kidney Injury, suspecting crystal nephropathy. The frequency of Acyclovir was reduced from thrice a day to once a day and on the fifth day of admission the serum creatinine was decreased to 2.25 mg/dl. Acyclovir was withdrawn and the patient was started on Tab Ganciclovir 450 mg twice a day IV hydration was given, nephrology opinion was taken before adjusting the dose of ganciclovir. Creatinine levels were checked on alternate days and they showed an improving trend after 24 hours of acyclovir withdrawal. The patient improved symptomatically, and the discharge medications included Acyclovir cream 5mg which was to be applied three times a day, and Tab. Valganciclovir 450 mg which was to be taken 2 times a day was prescribed for Zoster Infection.
Acyclovir-induced acute kidney injury is seen within 12-48 hours of administration. On the day of admission, the serum creatinine level was within normal range. The next day, the serum creatinine level elevated to 4.21 mg/dl following the injection of acyclovir (Figure 1). The creatinine levels gradually returned to normal once acyclovir was discontinued and Valganciclovir was started. Elevated creatinine levels suggest nephrotoxicity attributed to acyclovir, as cessation of acyclovir and initiation of valganciclovir resulted in a return to normal creatinine levels. Studies have shown that the side effect is more common when taken in the parenteral route compared to oral due to the speed and route of administration as the drug reaches the bloodstream sooner when compared to the oral route of administration. The side effect of acute kidney injury is usually correlated to the intravenous infusion of acyclovir [4].
Figure 1: Day-wise progress of serum creatinine levels.
Acyclovir Induced Acute Kidney Injury is a common side effect in most patients. Though the mechanism behind the Adverse Drug Reaction is unclear, it is important to check the renal function test before giving the drug as it may increase the possibility of side effects.
In this case report, the patient is given Acyclovir for Herpes infection, and the adverse reaction occurs within 24-48 hours. Withdrawal of the medication leads to resolving the reaction and the serum creatinine levels have come back to the normal range by the time the patient is discharged. Hence, it is important to monitor the creatinine levels when the drug is administered and ensure the normal condition of the patient.
The accomplishment of this research project would not have existed without the offerings and support of many things and institutions. grateful We are intensely grateful to all those who performed a function for the benefit of this project We too thank my mentors, Naweed Imam Syed, Prof. Department of Cell Biology at the University of Calgary, and Dr. Sadaf Ahmed Psychophysiology Lab, University of Karachi, for their priceless recommendations and support during the whole of this research. Their observations and knowledge assisted in forming the management concerning this project.
- Acyclovir (Oral Route, Intravenous Route) Description and Brand Names - Mayo Clinic. (n.d.). Www.mayoclinic.org. https://www.mayoclinic.org/drugssupplements/acyclovir-oral-route-intravenous-route/description/drg-20068393#:~:text=Acyclovir%20is%20used%20to%20treat
- Zachary KC. Acyclovir: An overview, UpToDate; 2022, https://www.uptodate.com/contents/acyclovir-an-overview
- Yildiz C, Ozsurekci Y, Gucer S, Cengiz AB, Topaloglu R. Acute kidney injury due to acyclovir. CEN Case Rep. 2013 May;2(1):38-40. doi: 10.1007/s13730-012-0035-0. Epub 2012 Oct 1. PMID: 28509218; PMCID: PMC5411519.
- Abuhelwa Z, Beran A, Venkataramany BS, Hinch BT, Assaly R. Concurrent Nephrotoxicity and Neurotoxicity Induced by Oral Valacyclovir in a Patient With Previously Normal Kidney Function. Cureus. 2022 Mar 31;14(3):e23693. doi: 10.7759/cureus.23693. PMID: 35509998; PMCID: PMC9060728.