More Information
Submitted: March 07, 2024 | Approved: March 28, 2024 | Published: March 29, 2024
How to cite this article: Abrantes LF, de Sousa JL, Ramos JMS, Leite RR, Ferreira SB. Correlation of Inappropriate use of Ceftriaxone and Bacterial Resistance in the Hospital Environment: Integrative Review. Arch Pharm Pharma Sci. 2024; 8: 014-020.
DOI: 10.29328/journal.apps.1001051
Copyright License: © 2024 Abrantes LF, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and repro-duction in any medium, provided the original work is properly cited.
Keywords: nappropriate use of antimicrobials; Ceftriaxone; Bacterial resistance
Correlation of Inappropriate use of Ceftriaxone and Bacterial Resistance in the Hospital Environment: Integrative Review
Larissa Furtado Abrantes1, Joyce Lima de Sousa1, Joel Messias Soares Ramos1, Rafael Rodrigues Leite1 and Sávio Benvindo Ferreira2*
1Medical Student in Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraíba, Brazil
2PhD in Pharmacology, Professor of Microbiology, Academic Unit of Life Sciences (UACV), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraíba, Brazil
*Address for Correspondence: Sávio Benvindo Ferreira, PhD in Pharmacology, Professor of Microbiology, Academic Unit of Life Sciences (UACV), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraíba, Brazil, Email: [email protected]
Introduction: Bacterial resistance is a threat to public health, as it is estimated that 37,000 people die due to hospital infections, most of them due to multidrug-resistant bacteria. In part, this resistance is due to the inappropriate use of antibiotics, with ceftriaxone being one of the most used. Therefore, this article aims to analyze the consequences of using ceftriaxone in the hospital environment.
Methodology: This is an integrative qualitative review, following the PICO strategy, using the Embase, BVS, and Pubmed databases, with the guiding question being: “In patients admitted to a hospital environment (P), is ceftriaxone used appropriately (I) for the treatment of infections (CO)?” and the time frame from 2013 to 2023.
Results: 272 articles were found in total, 46 obtained from the VHL, 62 from PubMed, and 164 from Embase. Of these, 66 were duplicates, leaving 206 works for title and summary reading. After reading, 79 were selected for full reading, with 7 articles ultimately being selected for the study. An average of 62.3% of inappropriate use was found, with the minimum value found being 19% and the maximum being 87.9%. The main reasons for this use were: indication, dose, frequency, and duration.
Conclusion: From reading the articles, it is concluded that the inappropriate use of ceftriaxone is mainly due to: indication, dose, frequency, and duration of treatment. These elements must be monitored, as their inappropriate use increases the length of hospital stay and may be associated with the emergence of bacterial resistance.
Bacterial resistance represents a serious threat to global public health, as it is estimated that approximately 37,000 people die from hospital-acquired infections, with the majority of these deaths being caused by multi-antibiotic-resistant bacteria [1]. The main factors that drive the persistence and dissemination of these microorganisms are the development of resistance mechanisms due to high selection pressure due to the inappropriate use of antibiotics; lack of management of broad-spectrum antibiotics; and lack of professional infection control services in the hospital environment [2]. Therefore, the rational use of antibiotics is essential to contain the development and spread of resistant bacteria in the hospital environment and in the community in general [3].
Thus, the inappropriate and excessive use of broad-spectrum antibiotics in the hospital environment, especially ceftriaxone, has been associated with the emergence of bacterial resistance and increased costs, due to improper prescription, essentially in empirical treatment, prolonged treatment duration and administration incorrect [4].
Ceftriaxone is an antibiotic from the third-generation cephalosporin class that is frequently used and can achieve an empirical prescription rate of 87% of cases [5]. This is because it has a broad spectrum of action, covering gram-positive and gram-negative bacteria, such as Streptococcus pneumoniae and Escherichia coli. onlineIn addition, it has a long half-life, as it is strongly associated with proteins, and can be administered once a day, which improves adherence to treatment, reduces side effects, and reduces costs, in order to benefit both patients and patients health systems [6]. A ceftriaxone is often prescribed to treat sepsis,as well as to combat urinary and respiratory infectionsand even cases of meningitis [7].
In view of the above, research is essential to assess the consequences of inappropriate use of antibiotics in the hospital environment in order to encourage the promotion of responsible use, preventing bacterial resistance, and improving the quality of medical care. In this sense, the research aims to evaluate, through an integrative review of prescription literature, the inappropriate use of ceftriaxone in hospitals.
Feature of the study
The present study is an integrative review of quantitative literature, which made use of scientific literature in order to discuss the topic investigated. Therefore, this research selected the most relevant articles on the subject in order to obtain a critical view of the issue addressed.
The search was based on the 6 steps for preparing an integrative review: definition of the research question; establishment of criteria for inclusion and exclusion of studies; definition of the information collected and categorization of the study; analysis of included studies; interpretation of results; and presentation of the review [8].
Conducting the investigation
The guiding question followed the PICO strategy, being the following: “In patients admitted to a hospital environment (P), is ceftriaxone used appropriately (I) for the treatment of infections (CO)?”. Next, a search was carried out in the following databases: Virtual Health Library (VHL), Embase, and Public Medlines (PubMed) of the National Library of Medicine, from 2013 to September 2023, selecting works in English, Portuguese, and Spanish.
The descriptors were selected from the Health Sciences Descriptors (DECS), in English for all bases, being: “ceftriaxone”, “bacterial resistance”, “drug resistance, microbial”, “inappropriate use” and “hospital”. These descriptors were grouped as follows to be the search formula in all databases: (‘ceftriaxone’/exp OR ceftriaxone) AND (‘bacterial resistance’/exp OR ‘bacterial resistance’ OR (bacterial AND (‘resistance’ /exp OR resistance)) OR ‘drug resistance, microbial’/exp OR ‘drug resistance, microbial’ OR ((‘drug’/exp OR drug) AND resistance, AND microbial)) AND (‘inappropriate use’ OR (inappropriate AND use)) AND (‘hospital’/exp OR hospital).
Selection parameters
The inclusion criteria adopted were: freely available studies, published in the last 10 (ten) years, from 2013 to 2023, in English, Portuguese, and Spanish. Regarding the exclusion criteria adopted, the following were excluded: book chapters, case reports, paid articles, duplicates, and studies that did not address the hospital environment or the use of ceftriaxone. Furthermore, the title and summary of all selected articles were analyzed and those that were not part of the established criteria were excluded. The remaining texts were read in full, and a new exclusion was made to obtain articles of real interest for the review.
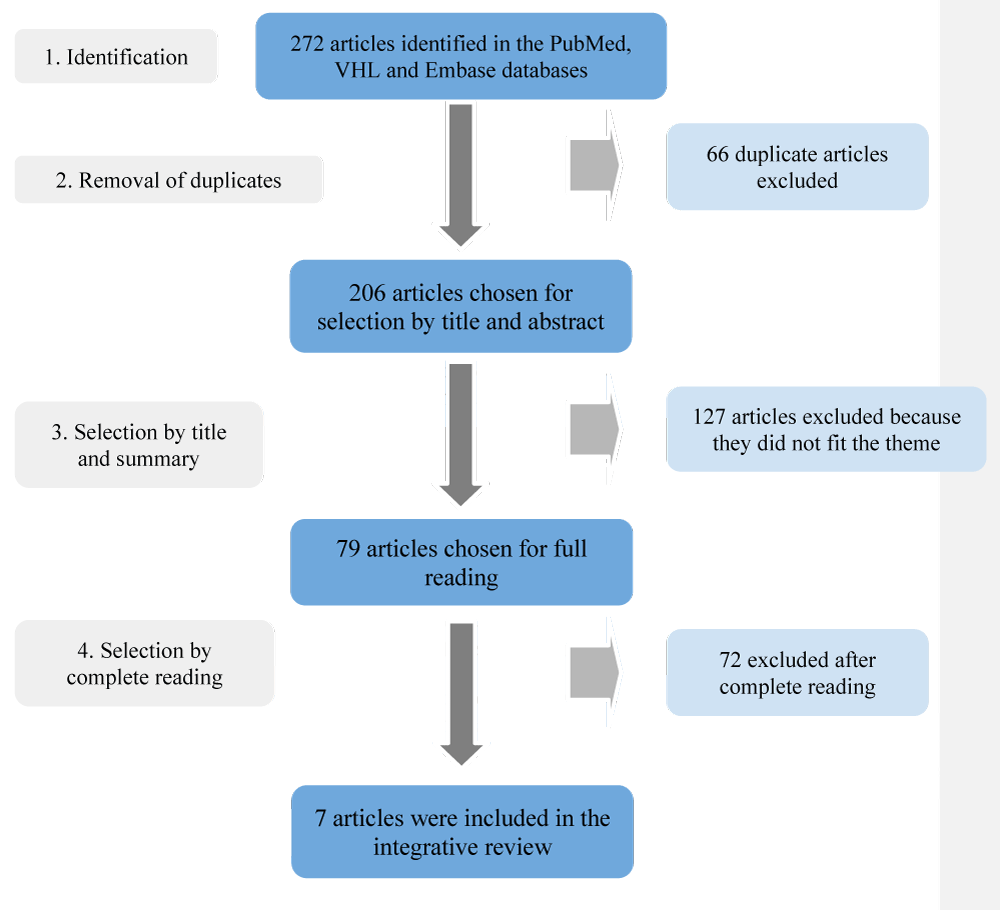
The research followed 4 steps, which are contained in the flowchart in Figure 1, named identification, removal of duplicates, selection by title and abstract, and selection by complete reading. A total of 272 articles were identified, 46 of which were obtained from the VHL, 62 from PubMed, and 164 from Embase. Of these, 66 were duplicates, generating 206 for title and abstract reading. As a result, 79 were chosen for complete reading and 72 were excluded because they did not fit the theme. Finally, only 7 articles provided quantitative data on the inappropriate use of ceftriaxone and were included in the integrative reading review table.
Figure 1: Selection of articles. Source: Own authorship, 2024. .
By reading the relevant articles, data were extracted to answer the guiding question, which was organized in Table 1 into the following fields: main author, article title, type of study, percentage of inappropriate use, percentage of inappropriate use, association with resistance, and completion.
| Table 1: Description of the articles included in the review, and analysis of the results regarding the inappropriateness of ceftriaxone and the association of bacterial resistance. | ||||||
| Main author | Country | Article title | Kind of study | Inappropriate use (%) | Did you associate it with resistance? | Conclusion |
| Bishaw [9] | Ethiopia | Appropriate Use of Ceftriaxone in Sub-Saharan Africa: A Systematic Review | Systematic review | NI | Yes | The review revealed that ceftriaxone was inappropriately prescribed to more than half of patients. |
| Sasi [15] | Tanzania | Ceftriaxone Prescription at Muhimbili National Hospital | Descriptive study | NI | Yes | Ceftriaxone is commonly prescribed inappropriately, and the risk of emergence and spread of ceftriaxone-resistant isolates may be high. |
| Gurtler [2] |
Switzerland |
Appropriateness of antimicrobial prescribing in a Swiss tertiary care hospital: a repeated point prevalence survey | Cross-sectional study | 19 | Yes | The proportion of inappropriate antimicrobial prescriptions was significant in a Swiss tertiary care center, which may contribute to or at least perpetuate the rise in antimicrobial resistance. |
| Ayele [10] | Ethiopia | Prospective evaluation of Ceftriaxone use in medical and emergency wards of Gondar university referral hospital, Ethiopia | Cross-sectional study | 80,2 | Yes | A very high rate of inappropriate use of ceftriaxone can increase the emergence of resistant organisms. |
| Ávila [18] | Chile | Changes in prescriptions and consumption of antimicrobials, the implementation of recommendations for use: experience in a university hospital | Prospective, interventional, quasi-experimental study | NI | Yes | Based on intervention measures implemented in a hospital, it was possible to evaluate the effect of use recommendations on reducing inappropriate prescriptions for ceftriaxone and levofloxacin. |
| Pallares [17] | Chile | Impact of Rational Use of Antibiotics in a Third level clinic in Colombia | Prospective quasi-experimental study | NI | Yes | With the results of the study, the importance of considering the construction and implementation of a strategy to combat the indiscriminate use of antimicrobials and bacterial resistance in Hospitals is highlighted. |
| Sileshi [19] | Ethiopia | Evaluation of ceftriaxone utilization in medical and emergency wards of Tikur Anbessa specialized hospital: a prospective cross-sectional study | Prospective cross-sectional study | 87,9 | Yes | Both the utilization rate and inappropriate use of ceftriaxone were very high in the medical and emergency wards of Tikur Anbessa Specialized Hospital. This can lead to the emergence of resistant pathogens which, in turn, compromise its effectiveness, leading to treatment failure and an increase in the cost of therapy. |
| Own authorship (2024). Caption: NI: Not Informed. | ||||||
Of the seven articles selected, one is from 2021, three are from 2019, one is from 2018, one is from 2017, and one is from 2016. Regarding the origin of the study: three of the works were carried out in Ethiopia, two in Chile, one in Tanzania, and one in Switzerland. Regarding the type of study, one systematic review, one descriptive study, three cross-sectional studies, and two quasi-experimental studies were included.
Four articles did not provide percentages of the total inappropriate use identified [15,17,18]. Only one of them revealed a percentage of adequate use [9], showing that the median of adequate use was 39.2%. Among the studies that provided statistical data exclusively on the inappropriate use of ceftriaxone [10], an average of 62.3% inappropriate use was observed, with values ranging from 19% to 87.9%. All studies showed that the practice of inappropriate prescription of ceftriaxone may be associated with an increased risk of emergence and dissemination of resistance.
In Table 2, it is possible to identify the main reasons for the inappropriate use of ceftriaxone.
| Table 2: Prevalence of circumstances that led to the inappropriate use of ceftriaxone in articles that indicated statistical data. | |||||
| Justification | Studies | ||||
| Bishaw [9] | Sasi [14] | Gurtler [2] | Ayele [10] | Sileshi [13] | |
| Recommendation | NI | 54% | 15% | 3,5% | 18,5% |
| Dose | 55% | 93% | 2,5% | 17,9% | 21% |
| Frequency | NI | 2,5% | 78,3% | 80,3% | |
| Duration | NI | 67% | 0% | 47% | 50% |
| Own authorship (2024). Caption: NI: Not Informed. | |||||
Inappropriate use of ceftriaxone
From the analysis of the results of the included articles, it was possible to verify an average of 62.3% of inappropriate use of ceftriaxone, with a maximum value reaching 87.9% (Table 1). This occurs mainly due to the high prevalence of ceftriaxone use in the hospital context, which is justified by its antibacterial potency, broad spectrum of activity, and low toxicity potential [11]. Studies show this trend in the use of ceftriaxone in several countries, with rates of 50.6% described in Uganda [12], 59% in Ethiopia [10], 49% in Australia [13], 20.51% in Tamil Nadu [14] and 11.4% in Eritrea [4].
The high use of ceftriaxone indicates an increase in errors in prescribing or even indicating the antibiotic. An average of 24.16% of indication errors was identified, so Sasi, et al. [15] observed that 54% of prescriptions were not indicated by the guidelines, given the lack of laboratories for microbiological analyses or the delay in obtaining the results [15]. Thus, the precise identification of the causative pathogen and the implementation of targeted treatment are hampered, causing the doctor to opt for a broad-spectrum medication, such as ceftriaxone, to carry out empirical therapy [4].
One of the tools that can be used to reduce errors is rational antimicrobial use programs, which emphasize the appropriate use of antimicrobials, prescribing them correctly, in appropriate doses and intervals to ensure the duration of treatment and the best route administration. Its objectives are: to optimize costs, prevent side effects associated with uncontrolled use, and avoid the development of resistant bacteria [16]. Based on this, evaluated changes in prescription patterns after implementing a program to rationalize the use of antimicrobials, resulting in a reduction in the consumption of ceftriaxone by 31%, which highlights the inappropriate use of this antimicrobial [17].
Furthermore, Ávila, et al. [11], after implementing recommendations for the use of ceftriaxone and levofloxacin, observed a reduction in the inappropriate use of antimicrobials by 35% in relation to the baseline value, which was 83% and significantly decreased to 53%. The indicators that showed the greatest variations were inappropriate indications and durations. It was observed that inappropriate indications decreased from 73.5% to 49.5%, varying from 33.3%. In relation to inadequate durations, the variation recorded was 69.7%, decreasing from 32.4% to 9.9% [18].
Found that inappropriate use of ceftriaxone was considerably higher in the emergency department than in medical wards (93.2% and 72.2% respectively), with a higher proportion of inappropriate use in the treatment of pneumonia and spontaneous bacterial peritonitis [10]. Sileshi, et al. [19] showed that the use of ceftriaxone was inappropriate in 87.9% of cases, mainly in medical wards and emergency rooms. The most frequent indication was for respiratory tract infections (35.4%), followed by skin, soft tissue, and bone infections (10.8%) [19]. The main error found was the frequency of dose administration (80.3%), followed by the lack of culture and sensitivity testing (53.2%) and inadequate duration of treatment (50%).
Furthermore, it was possible to observe a high rate of errors related to dose, frequency, and duration of treatment with ceftriaxone in this work, as described in Table 2. It is essential that the dose of any medication is correct, as well as its frequency of administration, in order to maintain bioavailability adequate for therapeutic success. Furthermore, such errors can result in treatment failure, toxicity, adverse effects, and the development of drug resistance, increasing costs for the healthcare system [15].
It is noteworthy that the lack of knowledge about treatment regimens and the deficiency in diagnostic competence contribute to inappropriate indications for ceftriaxone; administration of incorrect doses; lack of knowledge about adverse reactions and drug interactions; and sometimes the use of more expensive medications when less expensive medications would be equally or more effective [11].
It is worth highlighting that the difference in prevalence of some parameters analyzed in this study is due to the varied parameters used to evaluate correct or incorrect use. In Sasi, et al. [14], high indication errors were found because cases in which cultures were not performed were considered included in this parameter. In Ayele et al. [10], Gutler, et al. [2] and Sileshi et al. [19] this aspect was considered separately, with rates of 68.7%, 12.5%, and 18.5%, respectively [2,10,19]. Thus, the importance of this variable is evident, as quality metrics for antimicrobials include not only duration but also the selection of antimicrobials and the avoidance of excessively broad-spectrum antimicrobials in specific clinical circumstances [20].
Increased resistance to ceftriaxone
In view of the above regarding the misuse of ceftriaxone, it is worth mentioning that this situation tends to promote the exposure of microorganisms to insufficient concentrations of the drug, which can develop DNA encoding resistance, which promotes sensitization of bacteria, reducing the therapeutic effect on them [15]. This fact triggers an increase in the percentage of antimicrobial resistance to multiple antibiotics [21].
The acquisition of bacterial resistance is likely in the case of the antibiotic under analysis, as it is common for it to be prescribed widely and empirically, given its high potency against pathogens due to its broad spectrum, being used as a treatment for various bacterial infections [22]. As a result, bacterial resistance to ceftriaxone as well as a general increase in resistance to beta-lactams constitutes a high-level problem, as it makes the treatment ineffective, generating the need to seek other therapeutic alternatives, making it difficult to eliminate infectious diseases, such as pneumonia, bone infections, abdominal infections, skin and urinary tract, which have a high incidence, especially in developing countries, where there is a greater risk of a worse prognosis, which can lead to sepsis and death [4,10].
Other problems presented by the studies are the prolongation of the period of hospitalization in hospitals to treat the infection and a reduction in the therapeutic success rate of this drug. Zarauz, et al. [23] address this issue by analyzing that the indiscriminate use of antimicrobials could, by the year 2050, deprive them of their effectiveness in treating serious infections, based on the fact that Spain, as it ranks fifth in the world in outpatient prescriptions, has the number of 3000 people who die annually, as a result of bacterial infections due to antibiotic resistance [23].
Reinforcing this idea, the study by Ávila, et al. [11] showed that with the application of intervention measures in the use of antibiotics, the hospitalization period reduced from 19.8 ± 38.5 to 8.9 ± 7.2 days after -intervention while the cure rate increased, from 76.5% to 80.2%, reinforcing the need to apply more appropriate prescriptions. Furthermore, resistance to ceftriaxone has been related to an increased mortality rate from sepsis and septic shock in the hospital setting. This resistance occurred mainly in patients who had already been diagnosed with Chronic Obstructive Bronchopneumonia (COBP) and pneumonia [24]. There is consistent evidence in the literature that points to the frequent isolation of antimicrobial-resistant microorganisms among enteric gram negatives, including E. coli, Klebsiella species, Pseudomonas, and Serratia species, causing these serious infections [25]. This highlights the importance of prescribing antibiotics based on sensitivity tests.
Ayele, et al. [10] state that in 80.2% of cases the use of Ceftriaxone in her research was inappropriate, generating the hypothesis that this would enhance the emergence of resistant organisms. This is a reality faced by Eritrea, an African country, as it was seen that 62.5% of ceftriaxone therapy was inadequate, and it was noted that there was a pattern of Escherichia coli resistance in more than 50% to third-generation cephalosporins, including Ceftriaxone [4].
The study by Sasi, et al. [14] also shows that in addition to E. coli with a resistance rate of 63.5% to Ceftriaxone, other bacteria have high rates, such as coagulase-negative Staphylococcus, which presented around 79.5%, Klebsiella spp. 77.1% and Pseudomonas aeruginosa with 57.1%. This was also seen by Nusrat, et al. [26] at Chattogram Medical College Hospital in Bangladesh, where the ceftriaxone resistance rates of Klebsiella and Pseudomonas were 83% and 72% respectively [26]. Furthermore, studies by Altaf, et al. [27] in Pakistan showed a sensitivity of 9.5% of E. coli, 22.5% of Klebsiella pneumoniae and 23.8% Proteus mirabilis to ceftriaxone [27].
Some studies have also demonstrated an increase in the number of cases of Salmonella typhi resistant to third-generation cephalosporin antibiotics, mainly ceftriaxone, which is the first-line choice in empirical treatments (34%) in some cases. This bacteria is one of the main strains that causes multidrug-resistant (MDR) typhoid fever, and several countries have experienced outbreaks over the years, including Pakistan; India [28]; and China [29], among others.
Likewise, an increase in the resistance of bacteria isolated from the urinary tract to ceftriaxone was observed. Escherichia coli showed 72% resistance, the highest compared to other bacteria in the tract, due to its ability to produce extended-spectrum beta-lactamase (ESBL) that disrupts the beta-lactam ring, inactivating the beta-lactam antibiotic, seriously limiting therapeutic management. Other bacteria also showed resistance: Klebsiella pneumoniae 46.1% and Staphylococcus aureus 19% of the total bacterial isolates [21].
According to Souza, et al. [22], resistance to ceftriaxone has been increasing for a few years, one of these examples is Enterobacter cloacae, one of the main pathogens causing Healthcare-related Infections, causing severe pyelonephritis, meningitis of the newborn, endocarditis, brain abscesses, bacteremia, and sepsis [22,30]. According to studies carried out in France in 2004, the resistance of Enterobacter cloacae to ceftriaxone, between 1999 and 2002, increased, ranging from 24.3% to 29.6% (p = 0.03) [31], pointing out that resistance to ceftriaxone is not a recent problem, but rather a challenge that has developed over the years.
In that same study, an increase in resistance to ceftriaxone was related to the high biliary elimination of the drug compared to other drugs, thus showing its impact on the digestive flora, amplifying bla CTX-M resistance genes, as well as its ability toinactivate the cephalosporinase gene [32].
Mechanisms to combat bacterial resistance
Faced with this problem, it is necessary to understand the mechanisms that lead to its development and think of ways to combat them. Meletiadis, et al. [33] correlate increased resistance to ceftriaxone to two basic aspects: spread of epidemic multi-resistant strains through cross-transmission and the acquisition of resistance by susceptible strains. The same study relates increased resistance to therapies lasting longer than 14 days using ceftriaxone [33].
Therefore, several strategies have been studied to combat resistance to ceftriaxone. Some support the idea of developing new antibiotics, as the targets can be innovative and target completely different aspects of bacterial survival. Meanwhile, others maintain that microorganisms would likely develop resistance to these new drugs, emphasizing the need for more innovative and technological approaches in the formulation and distribution of existing drugs, as the prospect of overcoming antimicrobial resistance (AMR) through the development of new antibiotics have suffered a notable decline, especially in the case of gram-negative microorganisms [34,35].
This is largely due to the increasing likelihood of resistance to these new antibiotics to which pathogens can adapt in the same way as with previous antibiotics. As a result, emphasis has shifted to management programs, educational outreach primarily in the hospital setting, hygiene and disinfection interventions, the application of advanced formulations and delivery platforms, as well as the search for alternatives to traditional antibiotics [36]. Therefore, the implementation of antimicrobial management strategies has proven to be positive in combating antibiotic resistance, especially in the ICU environment. A decrease in resistance was observed in non-fermenting gram-negative bacteria, mainly Enterobacteriaceae, while, with regard to gram-positive bacteria, no significant changes were recorded [37].
Furthermore, a possible strategy is the development of vaccines to combat resistant strains. A study conducted in Taiwan observed that more than 90% of isolates that demonstrated resistance to ceftriaxone could be addressed by a conjugate vaccine. This suggests the feasibility of introducing pneumococcal conjugate vaccine in developing nations, especially in regions where resistance is prevalent [38].
Another approach that has been used is antimicrobial management, a set of practices and strategic interventions, which aim to address the problem of irrational use of antibiotics. These actions seek to reduce the excessive and empirical use of antibiotics to control bacterial resistance, prioritizing more prescriptions specific to pathologies [39]. According to Pallares and Catano [17], the application of interventions in the use of antibiotics interfered with the percentage of bacterial resistance to ceftriaxone of two bacteria analyzed in the study, however, the study observed relative maintenance for K. pneumoniae, going from 22% to 21 %, while for E. coli this number increased from 12% to 20%, relating these findings to the increased use of azithromycin and piperacillin [17]. Therefore, it is noted that the inappropriate use of one antibiotic can interfere with the resistance pattern of another, which alerts us to be careful with cross-resistance between antimicrobials.
Finally, the combination of nanoparticles with antibiotics emerges as a modern strategy to combat multidrug-resistant bacteria [40]. This method has the ability to inhibit several characteristics of bacterial resistance, including the active pumping of antibiotics out of bacterial cells, the development of bacterial biofilms, communication between bacterial cells through the quorum sensing system, and other related processes in cells microbial [41].
The present work shows that studies suggest an association between the inappropriate use of ceftriaxone and the emergence of bacterial resistance, as a consequence. Errors related to the indication, dose, frequency, and duration of treatment with ceftriaxone, as well as the empirical administration of this drug, precipitate the emergence of resistant infections, increasing the length of hospital stay and cases of sepsis and septic shock. Furthermore, studies show significant rates of resistance, with emphasis on E. coli, Klebsiella spp, and Pseudomonas aeruginosa, highlighting the importance of developing policies to manage antibiotic consumption in hospitals.
- da Silva RA. Antimicrobial Resistance: formulating the response in the context of global health. Health in Debate. 2020; 44: 607–623.
- Gürtler N, Erba A, Giehl C, Tschudin-Sutter S, Bassetti S, Osthoff M. Appropriateness of antimicrobial prescribing in a Swiss tertiary care hospital: a repeated point prevalence survey. Swiss Med Wkly. 2019 Oct 27;149:w20135. doi: 10.4414/smw.2019.20135. PMID: 31656037.
- Cabral L. Rationalization of antimicrobials in a hospital environment. Rev Soc Bras Clin Med. 2018.
- Berhe YH, Amaha ND, Ghebrenegus AS. Evaluation of ceftriaxone use in the medical ward of Halibet National Referral and teaching hospital in 2017 in Asmara, Eritrea: a cross sectional retrospective study. BMC Infect Dis. 2019 May 24;19(1):465. doi: 10.1186/s12879-019-4087-z. PMID: 31126242; PMCID: PMC6534921.
- Barman M, Al Hariri B, Rahman Mustafa A, Ambra N, Amjed I, Eid Nazzal Alharafsheh A, Illahi MN, Hamuda S, Gaafar M, Sharif M. Ceftriaxone-induced hepatotoxicity in patients with common medical infections in Qatar: A retrospective study. Qatar Med J. 2022 Jul 7;2022(3):27. doi: 10.5339/qmj.2022.27. PMID: 35864919; PMCID: PMC9272765.
- Tevyashova AN, Korolev AM, Mirchink EP, Isakova EB, Osterman IA. Synthesis and evaluation of biological activity of benzoxaborole derivatives of azithromycin. J Antibiot (Tokyo). 2019 Jan;72(1):22-33. doi: 10.1038/s41429-018-0107-2. Epub 2018 Oct 12. PMID: 30315257.
- Zanoni DR. The occurrence of adverse events with the use of Ceftriaxone. Brazilian Journal of Health Review. 2023; 6: 4220-4234.
- Dehkordi AH. How to write a systematic review: A narrative review. International Journal of Preventive Medicine. 2021; 12.
- Meresa Bishaw B, Tegegne GT, Berha AB. Appropriate Use of Ceftriaxone in Sub-Saharan Africa: A Systematic Review. Infect Drug Resist. 2021 Aug 28;14:3477-3484. doi: 10.2147/IDR.S329996. PMID: 34483671; PMCID: PMC8409767.
- Ayele AA, Gebresillassie BM, Erku DA, Gebreyohannes EA, Demssie DG, Mersha AG, Tegegn HG. Prospective evaluation of Ceftriaxone use in medical and emergency wards of Gondar university referral hospital, Ethiopia. Pharmacol Res Perspect. 2018 Feb;6(1):e00383. doi: 10.1002/prp2.383. PMID: 29417764; PMCID: PMC5817827
- Menkem EZ. Attitudes and Practices of the Use of Third-Generation Cephalosporins among Medical Doctors Practicing in Cameroon. International Journal of Clinical Practice. 2023; 1-7.
- Kizito M, Lalitha R, Kajumbula H, Ssenyonga R, Muyanja D, Byakika-Kibwika P. Antibiotic Prevalence Study and Factors Influencing Prescription of WHO Watch Category Antibiotic Ceftriaxone in a Tertiary Care Private Not for Profit Hospital in Uganda. Antibiotics (Basel). 2021 Sep 26;10(10):1167. doi: 10.3390/antibiotics10101167. PMID: 34680748; PMCID: PMC8532977.
- Friedman D. OUP accepted manuscript. JAC-Antimicrobial Resistance. 2020; 2.
- Prakasam A. A Prospective Antimicrobial Prescription Audit in the Inpatient Department of Pulmonology in a Tertiary Care Hospital. International Journal of Life Science and Pharmaceutical Research. 2022; 10.
- Sasi PG. Ceftriaxone Prescription at Muhimbili National Hospital. Tanzania Journal of Health Research. 2019; 21: 1-13.
- SATO, Silvia Akemi. Evaluation of rational use of antimicrobial programs in hospitals in the state of São Paulo. 2019. Doctoral Thesis. University of São Paulo.
- Pallares CJ, Cataño JC. Impact of the rational use of antimicrobials in a third-level clinic in Colombia. Chilean Journal of Infectology. 2017; 34: 205-211.
- Ávila F, Luppi M, Gaete P, Rivas A, Silva F, Olivares R. Cambios en las prescripciones y el consumo de antimicrobianos, luego de la implementación de recomendaciones de uso: experiencia en un hospital universitario [Changes in prescriptions and antibiotic consumption after the implementation of recommendations for use: experience in a university hospital]. Rev Chilena Infectol. 2019 Jun;36(3):253-264. Spanish. doi: 10.4067/S0716-10182019000300253. PMID: 31859743.
- Sileshi A, Tenna A, Feyissa M, Shibeshi W. Evaluation of ceftriaxone utilization in medical and emergency wards of Tikur Anbessa specialized hospital: a prospective cross-sectional study. BMC Pharmacol Toxicol. 2016 Feb 18;17:7. doi: 10.1186/s40360-016-0057-x. PMID: 26891697; PMCID: PMC4759859.
- Delorme C, Viel-Thériault I, Moradipour T, Le Saux N. Drug use evaluation (DUE) of ceftriaxone: A quality metric in a pediatric hospital. J Assoc Med Microbiol Infect Dis Can. 2020 Oct 11;5(3):139-144. doi: 10.3138/jammi-2019-0026. PMID: 36341311; PMCID: PMC9608729.
- Gashe F, Mulisa E, Mekonnen M, Zeleke G. Antimicrobial Resistance Profile of Different Clinical Isolates against Third-Generation Cephalosporins. J Pharm (Cairo). 2018 Sep 9;2018:5070742. doi: 10.1155/2018/5070742. PMID: 30271652; PMCID: PMC6151245.
- Souza M. Ceftriaxone: rational use by the Pediatric’s department of the Santa Casa’s Hospital of Belo Horizonte, MG. Pediatric Residency. 2020; 10.
- Zarauz JM, Zafrilla P, Ballester P, Cerda B. Study of the Drivers of Inappropriate Use of Antibiotics in Community Pharmacy: Request for Antibiotics Without a Prescription, Degree of Adherence to Treatment and Correct Recycling of Leftover Treatment. Infect Drug Resist. 2022 Nov 23;15:6773-6783. doi: 10.2147/IDR.S375125. PMID: 36447792; PMCID: PMC9701454.
- Wongsurakiat P, Chitwarakorn N. Severe community-acquired pneumonia in general medical conditions: outcomes and impact of initial antibiotic selection. BMC Pulmonary Medicine. 2019; 19.
- Van Besien RF, Hampton N, Micek ST, Kollef MH. Ceftriaxone resistance and adequacy of initial antibiotic therapy in community onset bacterial pneumonia. Medicine (Baltimore). 2022 May 20;101(20):e29159. doi: 10.1097/MD.0000000000029159. PMID: 35608417; PMCID: PMC9276381.
- Nusrat T, Akter N, Rahman NAA, Godman B, D Rozario DT, Haque M. Antibiotic resistance and sensitivity pattern of Metallo-β-Lactamase Producing Gram-Negative Bacilli in ventilator-associated pneumonia in the intensive care unit of a public medical school hospital in Bangladesh. Hosp Pract (1995). 2020 Aug;48(3):128-136. doi: 10.1080/21548331.2020.1754687. Epub 2020 May 6. PMID: 32271642.
- Altaf U, Saleem Z, Akhtar MF, Altowayan WM, Alqasoumi AA, Alshammari MS, Haseeb A, Raees F, Imam MT, Batool N, Akhtar MM, Godman B. Using Culture Sensitivity Reports to Optimize Antimicrobial Therapy: Findings and Implications of Antimicrobial Stewardship Activity in a Hospital in Pakistan. Medicina (Kaunas). 2023 Jul 2;59(7):1237. doi: 10.3390/medicina59071237. PMID: 37512049; PMCID: PMC10384799.
- Samajpati S, Pragasam AK, Mandal S, Balaji V, Dutta S. Emergence of ceftriaxone resistant Salmonella enterica serovar Typhi in Eastern India. Infect Genet Evol. 2021 Dec;96:105093. doi: 10.1016/j.meegid.2021.105093. Epub 2021 Sep 27. PMID: 34592414.
- Yan M, Li X, Liao Q, Li F, Zhang J, Kan B. The emergence and outbreak of multidrug-resistant typhoid fever in China. Emerg Microbes Infect. 2016 Jun 22;5(6):e62. doi: 10.1038/emi.2016.62. PMID: 27329848; PMCID: PMC4932652.
- Pang F, Jia XQ, Zhao QG, Zhang Y. Factors associated to prevalence and treatment of carbapenem-resistant Enterobacteriaceae infections: a seven years retrospective study in three tertiary care hospitals. Ann Clin Microbiol Antimicrob. 2018 Mar 23;17(1):13. doi: 10.1186/s12941-018-0267-8. PMID: 29571291; PMCID: PMC5865290.
- Souna D, Drissi M, Almahmoud I, Maurin M. Enterobacter cloacae Complex and CTX-M Extended-Spectrum β-Lactamases in Algeria. Microb Drug Resist. 2022 Mar;28(3):346-354. doi: 10.1089/mdr.2020.0535. Epub 2021 Dec 10. PMID: 34890283.
- Ching C, Orubu ESF, Sutradhar I, Wirtz VJ, Boucher HW, Zaman MH. Bacterial antibiotic resistance development and mutagenesis following exposure to subinhibitory concentrations of fluoroquinolones in vitro: a systematic review of the literature. JAC Antimicrob Resist. 2020 Sep 30;2(3):dlaa068. doi: 10.1093/jacamr/dlaa068. PMID: 34223024; PMCID: PMC8210091.
- Meletiadis J, Turlej-Rogacka A, Lerner A, Adler A, Tacconelli E, Mouton JW; the SATURN Diagnostic Study Group. Amplification of Antimicrobial Resistance in Gut Flora of Patients Treated with Ceftriaxone. Antimicrob Agents Chemother. 2017 Oct 24;61(11):e00473-17. doi: 10.1128/AAC.00473-17. Erratum in: Antimicrob Agents Chemother. 2018 Nov 26;62(12): PMID: 28807914; PMCID: PMC5655041.
- Tewabe A, Marew T, Birhanu G. The contribution of nano-based strategies in overcoming ceftriaxone resistance: a literature review. Pharmacol Res Perspect. 2021 Aug;9(4):e00849. doi: 10.1002/prp2.849. PMID: 34331383; PMCID: PMC8324973.
- Hrvatin V. Combating antibiotic resistance: New drugs or alternative therapies? CMAJ. 2017 Sep 18;189(37):E1199. doi: 10.1503/cmaj.109-5469. PMID: 28923803; PMCID: PMC5602506.
- Alanazi M, Alqahtani HM, Alshammari MK, Alshammari RM, Malik JA, Ahmed S, Aroosa M, Shinde M, Alharby TN, Ansari M, Hussain A, Alkhrshawy FF, Anwar S. Infection Prevalence at a Tertiary Hospital in Hail, Saudi Arabia: A Single-Center Study to Identify Strategies to Improve Antibiotic Usage. Infect Drug Resist. 2023 Jun 13;16:3719-3728. doi: 10.2147/IDR.S413295. PMID: 37333682; PMCID: PMC10276591.
- Bouharkat B, Tir Touil A, Mullié C, Chelli N, Meddah B. Bacterial ecology and antibiotic resistance mechanisms of isolated resistant strains from diabetic foot infections in the north west of Algeria. J Diabetes Metab Disord. 2020 Sep 30;19(2):1261-1271. doi: 10.1007/s40200-020-00639-5. PMID: 33553027; PMCID: PMC7843819.
- Shakya AK, Al-Sulaibi M, Naik RR, Nsairat H, Suboh S, Abulaila A. Review on PLGA Polymer Based Nanoparticles with Antimicrobial Properties and Their Application in Various Medical Conditions or Infections. Polymers (Basel). 2023 Aug 30;15(17):3597. doi: 10.3390/polym15173597. PMID: 37688223; PMCID: PMC10490122.
- de Melo RC.. Management of interventions to prevent and control antimicrobial resistance in hospitals: evidence review. Revista Panamericana de Salud Pública. 2020; 44.
- Baptista PV. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans. Frontiers in Microbiology. 2018; 9.
- Himanshu, Mukherjee R, Vidic J, Leal E, da Costa AC, Prudencio CR, Raj VS, Chang CM, Pandey RP. Nanobiotics and the One Health Approach: Boosting the Fight against Antimicrobial Resistance at the Nanoscale. Biomolecules. 2023 Jul 28;13(8):1182. doi: 10.3390/biom13081182. PMID: 37627247; PMCID: PMC10452580