More Information
Submitted: March 07, 2024 | Approved: May 01, 2024 | Published: May 02, 2024
How to cite this article: Fonseca PEO, Azevedo JA, Bié SMG, Ferreira SB. Benefits of using SLGT2 Inhibitors for Patients with CDK and DM2 to Reduce Mortality Risks. Arch Pharm Pharma Sci. 2024; 8: 041-046.
DOI: 10.29328/journal.apps.1001055
Copyright License: © 2024 Fonseca PEO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and repro-duction in any medium, provided the original work is properly cited.
Keywords: SLGT2 inhibitors; Chronic kidney disease; T2DM
Benefits of using SLGT2 Inhibitors for Patients with CDK and DM2 to Reduce Mortality Risks
Pandora Eloa Oliveira Fonseca1, Jeremias Aguiar Azevedo1, Sara Maria Gomes Bié1 and Sávio Benvindo Ferreira2*
1Medical student, Academic Unit of Life Sciences (UACV), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraíba, Brazil
2PhD in Pharmacology, Academic Unit of Life Sciences (UACV), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraíba, Brazil
*Address for Correspondence: Sávio Benvindo Ferreira, PhD in Pharmacology, Academic Unit of Life Sciences (UACV), Teacher Training Center (CFP), Federal University of Campina Grande (UFCG), 58900-000, Cajazeiras, Paraíba, Brazil, Email: [email protected]
Type 2 diabetes mellitus (T2DM) is the most common cause of chronic kidney disease (CKD). CKD is characterized by progressive liver tissue damage and is an important risk factor for mortality due to renal and cardiovascular outcomes. Thus, randomized clinical trials have investigated the use of sodium-glucose cotransporter 2 (SLGT2) inhibitors as a promising therapy for patients with CKD and T2DM. This study aimed to analyze the benefits of using SGLT2 inhibitors in patients with CKD and T2DM to reduce mortality risks. To this end, a qualitative, descriptive methodological approach was adopted using a literature review in the PubMed, Embase, and VHL databases. The inclusion criteria were clinical trial articles, randomized or non-randomized, cohort studies, case-control studies, and open access, published in Portuguese and English, between 2018 and 2023 with topics associated with SGLT2 inhibitors, CDK, and T2DM patients. In this context, it was observed that the risk of death from CKD in patients treated with Canaglifozin was 30% lower than in those treated with a placebo and that Dapaglifozin prolonged survival. In this context, when assessing the progression of kidney disease or death from cardiovascular causes in patients taking Empagliflozin, only 13.1% achieved the outcome compared to 16.9% on placebo, so the drug safely reduces the risk of mortality. Consequently, SGLT2 inhibitors have shown excellent results in the treatment of CDK and T2DM, with a reduction in the risk of mortality, positive effects on reducing renal and cardiovascular outcomes, as well as prolonging survival.
Chronic kidney disease (CKD) is characterized by progressive and irreversible damage to the liver tissue, which leads to loss of functionality of the organ, i.e. loss of ability to control the hydro electrolyte balance, regulation of blood pressure, and elimination of toxins from the body. Thus, the most advanced stage of the loss of kidney function seen in CKD consists of the accumulation of substances toxic to the body in the blood due to non-filtration [1-4].
According to data from the Brazilian Society of Nephrology, 7.25% of individuals over the age of 30 and 28% to 46% of individuals over the age of 46 in the world have CKD, with more than 10 million in Brazil alone. It is also associated with two chronic diseases with a high incidence in the Brazilian population: Diabetes and Hypertension.
The Diabetes Atlas of the International Diabetes Federation (IDF) reveals that Brazil ranks fifth in the world for the incidence of diabetes in the population, with 16.8 million sufferers. Diabetes is one of the main causes of CKD, with 25% of patients with type 1 diabetes and 10% of those with type II diabetes developing chronic kidney disease. Diabetic nephropathy is also a complication of DM that mainly affects patients with DM2 [2,5,6].
The relevance of this research is based on the growing prevalence of type 2 diabetes in the Brazilian and world population since it is a predisposing factor for chronic kidney disease and few treatments that can be used in the long term are available. Thus, in cardiovascular trials of sodium-glucose cotransporter 2 (SGLT2) inhibitors, the results suggested that they may improve renal outcomes in patients with DM2 [3,7,8].
Sodium-glucose cotransporter (SGLT2) inhibitors are diabetic drugs that act by inhibiting glucose reabsorption in the proximal renal tubule, their inhibition causing glucose loss in the urine and a decrease in serum levels [4]. The main examples of SGLT2 inhibitors are canagliflozin, dapagliflozin, and empagliflozin, which have a direct relationship with reducing the risk of hospitalization and the development of chronic kidney disease. In line with the above, of the drugs used in patients with diabetes, only SGLT2 inhibitors have been shown to have a significant cardioprotective effect, due to greater osmotic diuresis as a result of the greater excretion of glucose in the urine [5,9-11].
Given the above, this research aims to analyze the benefits of using SLGT2 inhibitors for patients with chronic kidney disease and DM2 to reduce the risk of mortality, to raise a discussion about the insertion of these drugs in clinical practice and improvements in the prognosis of chronic patients in the country.Characterization of the research
The production of a literature review enables the construction of knowledge on a given subject in the health area, which directly contributes to the identification of gaps in the literature, highlighting the need for further research or recommendations for clinical practice [6,12,13,15]. This article is characterized by a bibliographic and descriptive literature review, which aims to analyze the benefits of using SLGT2 inhibitors in patients with chronic kidney disease and DM2.
Conducting research
The search was carried out using the Pubmed, Embase, and Virtual Health Library databases, with the guiding question being “What are the benefits of using SLGT2 inhibitors for patients with chronic kidney disease and type 2 diabetes mellitus?”.
The database was searched using the following Health Science Descriptors (DeCS): “SLGT-2 inhibitor”, “Sodium-glucose Cotransporter-2 Inhibitors”, “Type 2 diabetes”, “Chronic Kidney disease”, “Death”, using the Boolean operators “AND” and “OR”. Thus, the search strategy used in all the databases was: (“SLGT-2 inhibitor” OR “canagliflozin” OR “dapagliflozin” OR “empagliflozin” OR “Sodium-glucose Cotransporter-2 Inhibitors”) AND (“DM2” OR “type 2 diabetes”) AND (“chronic kidney disease”) AND(“death” OR “mortality”).
Criteria selection
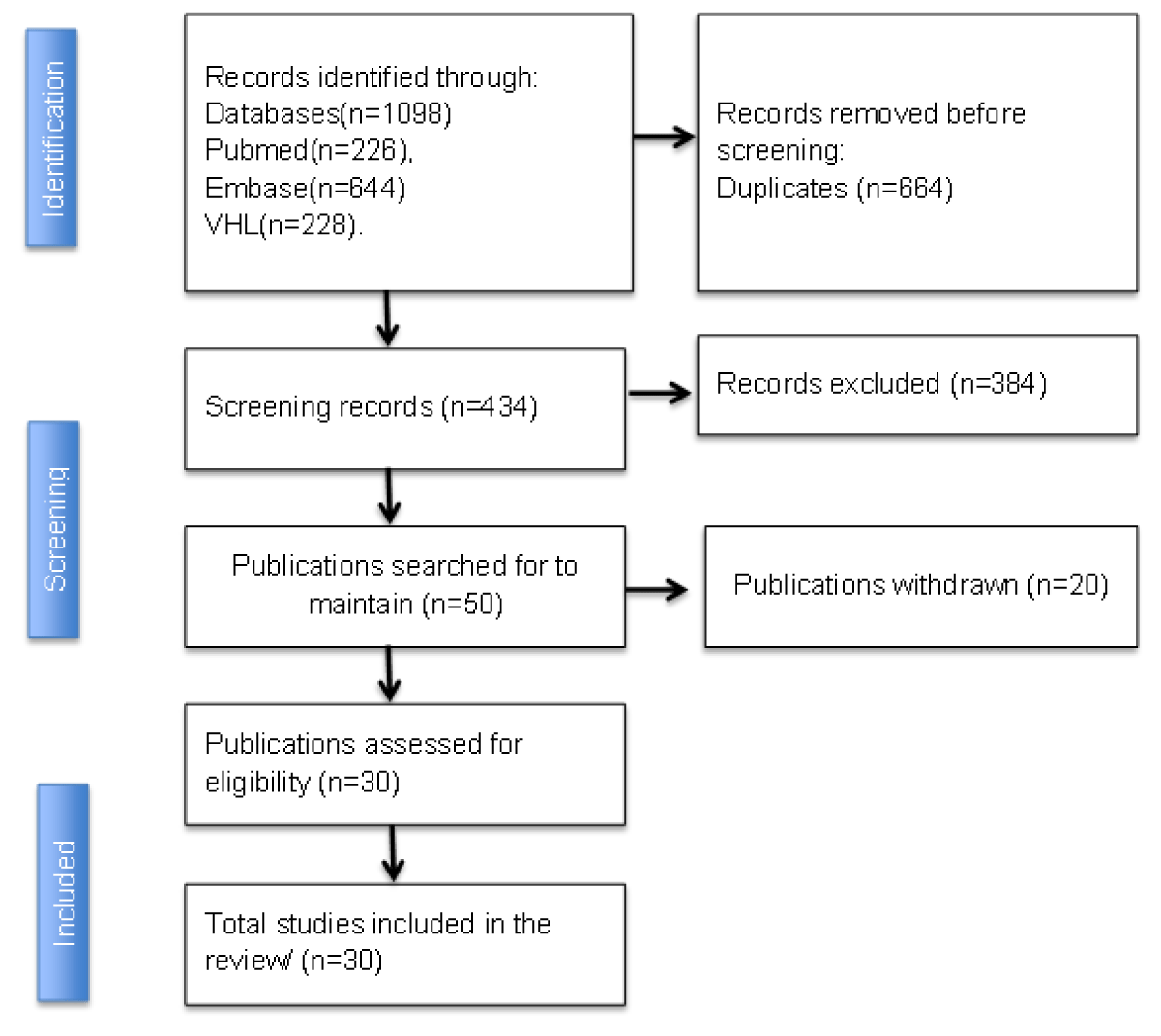
The inclusion criteria used were: articles published in Portuguese and English, between 2018 and 2023, with full text available, so that the articles included were clinical trials, randomized or non-randomized, cohort studies, case-control studies, open access, with topics associated with SGLT2 inhibitors, chronic kidney patients and type 2 diabetics. The exclusion criteria were duplicate studies, editorials and reviews, animal studies, and those that were not freely available to read the full text. The search resulted in 1098 articles in total, distributed as follows: PubMed (n:226); Embase (n:644), Virtual Health Library (n:228). To facilitate the exclusion of duplicates and the inclusion of articles that would make up the sample, selected based on the criterion of relevance to the topic, the free Rayyan platform was used, which consists of a free online application/website to help researchers with the methodology of literature reviews, systematic reviews and/or meta-analyses. Of the 1098 articles, 664 were duplicated, 614 were excluded and 50 were included; the final sample consisted of 30 articles. The figure below illustrates the selection and eligibility check of the articles to make up the review.
Presentation of results and summary of information
After reading and applying the criteria for relevance to the topic, information was collected that met the aim of the article, and the findings were discussed in a dissertation.
Based on the bibliographical research, after associating all the descriptors in the databases searched, 1098 articles were found. All the articles were found in the Embase, PubMed, and Virtual Health Library (VHL) databases. After applying the inclusion and exclusion criteria, 50 full-text articles were selected and assessed for eligibility. Of this total, 20 articles were excluded because they did not have an outcome, drug, or condition that met the thematic delimitation required for discussion.
Of these 20 excluded studies, the main themes found were the use of SLGT2 inhibitors in diabetic patients without chronic kidney disease, only as a hypoglycemic drug, as well as articles that reported other types of drugs such as SLGT1 inhibitors and SLGT1 combined with SLGT2. This brings the total for the complete analysis to 30 articles, as shown in Figure 1.
Figure 1: Flowchart for identifying and selecting the articles selected from the Embase, PubMed and VHL database.
Ethical aspects
It was not necessary to submit this study to the Research Ethics Committee, as it is a review of the available academic literature.
Sodium-glucose cotransporter 2 (SLGT2) inhibitors
Sodium-glucose cotransporter 2 (SLGT2) inhibitors were developed to treat type 2 diabetes mellitus by inhibiting glucose reabsorption in the proximal tubule [7]. Their use has increased significantly in recent years as they show great benefits in renal and cardiovascular outcomes [8].
Thus, it represents a new drug therapy for patients with CKD, in which the main renoprotective mechanism occurs through physiological modulation by tubuloglomerular feedback, with the inhibition of proximal tubular sodium reabsorption and, subsequently, a reduction in intraglomerular hypertension by afferent arteriolar vasoconstriction [9]. Consequently, this constriction reduces progressive liver damage [10,14,16,17].
The hypoglycemic mechanism occurs in an insulin-dependent manner and contributes to improved pancreatic beta cell function and greater insulin sensitivity. In addition, the cardioprotective effect is reported to reduce stress on the heart wall, which reduces the progression of heart failure. SGLT2 inhibitors are also associated with maximizing glycosuria, which helps with weight loss [11,18,19-21].
Use of SLGT2 inhibitors in Type 2 diabetic patients with chronic kidney disease
From this perspective, several studies were analyzed to better understand the subject of the study. In the randomized clinical trial called EMPA-KIDNEY, 6,609 patients were randomly assigned to receive the SLGT-2 inhibitor empagliflozin versus placebo to assess the progression of chronic kidney disease. Of these, 46% were diabetic, 44% type 2, and 56% type 1. Furthermore, when evaluating the progression of chronic kidney disease or death due to cardiovascular outcomes, 13.1% of the empagliflozin group suffered a death, while patients on placebo therapy suffered 16.9%, i.e. empagliflozin had no significant effect on the progression of the case to death. Thus, empagliflozin safely reduces the risk of mortality [12,22-25].
In addition, in the randomized, double-blind CREDENCE study, DM2 patients with chronic kidney disease were assigned to receive canagliflozin. In this trial, 4401 patients underwent randomization with a follow-up of 31.5 months. The data was positive for the canagliflozin group, where there was a lower risk of death from kidney causes or cardiovascular disease (30%), kidney death (34%), cardiovascular death or myocardial infarction or stroke (80%), and hospitalization for heart failure (61%). The relative risk of end-stage renal disease, doubling of serum creatinine level, or renal death was lower by 34% in the canagliflozin group [13,26-30].
Similarly, studies have shown that dapagliflozin was able to reduce the risk of adverse events such as kidney and heart failure and mortality by 32% in diabetic patients with chronic kidney disease. Therefore, the reduction in mortality may be related to increased survival in CKD patients with or without DM2 taking dapaglifozin [7,14,31,32,33-35].
In the study carried out [15,36-39]. Patients predisposed to developing severe stages of chronic kidney disease were randomized into a placebo group and a dapagliflozin group to assess the efficacy of the drug in reducing the progression of the disease. Thus, dapagliflozin, even in patients at very high risk of end-stage chronic disease, proved to be effective and safe, reducing the development of the disease and cardiac or renal death by up to 50%.
Other observational evidence has suggested the beneficial effects of sodium-glucose inhibitors (canagliflozin, dapagliflozin, and empagliflozin), showing a 35% reduction in the incidence of hospitalization for heart failure due to treatment with empagliflozin. In addition to the effects on the cardiovascular system, macroalbuminuria, a doubling of the serum creatinine level, was significantly reduced by 39%, which results in improved cardiovascular and renal outcomes, reducing the mortality rate in people with type 2 diabetes (DM2) [16,40-44].
In light of the above, treatment with SGLT2 inhibitors represents a promising therapeutic option for CKD patients with DM2 or without DM2 based on a reduction in mortality due to cardiovascular and renal outcomes.
Benefits of using SLGT2 inhibitors
The drugs in question are easily absorbed after oral administration and quickly reach maximum concentration within two hours. Due to their insulin-independent mechanism of action, they can be the therapy of choice at any stage of the disease’s progression, with the advantages of reducing glycated hemoglobin levels, lowering fasting and post-meal blood glucose, reducing weight, and cardiovascular and renal outcomes [17,15,45-47].
In addition to their effects described in the treatment of hyperglycemia in DM2 and chronic kidney disease, their diuretic effect, in addition to generating an improvement in the glycemic profile, is also seen in cardiac output, which has a positive impact on cardiac function. Accordingly, these drugs are effective in weight loss and in regulating systemic blood pressure, which has a positive impact on cardiovascular health [19,48,49].
In patients with chronic kidney disease and DM2, a decrease in albuminuria was observed with the use of these drugs, which allows them to be correlated with a slowdown in the progressive loss of kidney function [20,50-52].
SLGT2 inhibitors are drugs that have significant cardioprotective effects, for example, Empagliflozin, Dapagliflozin, and Canagliflozin. These drugs are now widely approved therapies because of their osmotic diuretic and natriuretic effects contributing to reduced plasma volume and having a significant effect on decreasing systolic and diastolic blood pressures by 4 to 6 and 1 to 2 mm Hg, respectively. The EMPA-REG OUTCOME trial in patients with T2DM and cardiovascular disease randomly assigned to empagliflozin versus placebo reported a 14% reduction in the primary composite outcome of cardiovascular death, nonfatal stroke, nonfatal myocardial infarction and reduced 30% cardio-vascular mortality and heart failure hospitalizations [53].
Adverse effects of SGLT2 inhibitors
It is worth noting that there are reservations regarding the prescription of these drugs, given the mechanism of action of these drugs, which consists of their action of reducing the tubular reabsorption of glucose, leading to glycosuria, which generates an increase in the production of ketone bodies, which is due to the reduction of the anti-lipolytic action of insulin, this mechanism justifies a condition of diabetic euglycemic ketoacidosis (CED) [54]. According to research, there are, on average, 6 cases of CED for every 1000 patients using this pharmacological class [55].
Furthermore, given its action on the urinary tract, the use of SGLT2 has also been associated with an increased occurrence of urinary tract infections, as glucose concentrations occur in the urethra and ureters. Furthermore, it increases the rate of bone fractures, and increases hematocrit, among other effects that must be taken into consideration before prescribing [56].
Based on an analysis of the various studies found in the literature, SGLT2 inhibitors and sotagliflozin can be considered a major pharmacological advance in a wide variety of areas, especially nephrology and cardiology, given their excellent results in the treatment of chronic kidney disease and type 2 diabetes mellitus, bringing hope and a new perspective on quality of life for patients affected by these and various other pathologies, with a reduction in the risks associated with mortality in these individuals. Therefore, SGLT2 inhibitors faster than other drugs in the market have rapidly become a first-line treatment for CKD and T2MD patients, future researchers should investigate if SGLT2 inhibitors could be used to prevent the development of CKD in patients with diabetes besides reducing mortality risks, renal and cardiovascular outcomes. Thus, additional subanalyses and more specific clinical trials are awaited to settle unconvincing available evidence.
- Daiana DB, Campos R. Chronic renal failure and its implications for metabolic systems. Uniandrade Magazine. 2017; 18:149–156. rev.uniandrade.br/index.php/revistauniandrade/article/view/691, https://doi.org/10.5935/revuniandrade.v18i3.691.
- da Silva TK. Diabetes Mellitus and Arterial Hypertension in Patients with Chronic Renal Failure on Dialysis. Integrative Review. Research, Society and Development. 2021; 10: e53410616121. https://doi.org/10.33448/rsd-v10i6.16121.
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. New England Journal of Medicine [Internet]. 2019 Jun 13;380(24):2295–306.
- Saisho Y. SGLT2 Inhibitors: The Star in the Treatment of Type 2 Diabetes? Diseases. 2020; 8: 14. https://doi.org/10.3390/diseases8020014.
- Almeida CIF de. Impact of adverse effects of oral antidiabetics on adherence to therapy and quality of life in type 2 Diabetes Mellitus [Internet]. common.rcaap.pt. 2019 [cited 2024 Apr 28]. Available from: http://hdl.handle.net/10400.26/32923
- Mende CW. Chronic Kidney Disease and SGLT2 Inhibitors: A Review of the Evolving Treatment Landscape. Adv Ther. 2022 Jan;39(1):148-164. doi: 10.1007/s12325-021-01994-2. Epub 2021 Nov 30. PMID: 34846711; PMCID: PMC8799531.
- Lo KB, Gul F, Ram P, Kluger AY, Tecson KM, McCullough PA, Rangaswami J. The Effects of SGLT2 Inhibitors on Cardiovascular and Renal Outcomes in Diabetic Patients: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2020;10(1):1-10. doi: 10.1159/000503919. Epub 2019 Nov 19. PMID: 31743918.
- Sridhar VS, Dubrofsky L, Boulet J, Cherney DZ. Making a case for the combined use of SGLT2 inhibitors and GLP1 receptor agonists for cardiorenal protection. J Bras Nefrol. 2020 Oct-Dec;42(4):467-477. doi: 10.1590/2175-8239-JBN-2020-0100. PMID: 32926067; PMCID: PMC7860654.
- Silva PT. Use of SGLT2 Inhibitors as a New Drug Therapy in the Treatment of Chronic Kidney Disease in Patients with Diabetes Mellitus / Use of SGLT2 Inhibitors as a New Drug Therapy in the Treatment of Chronic Kidney Disease in Diabetes Mellitus Patients. Brazilian Journal of Development. 2022; 8: 34366–34381.
- Bezerra TG, Gonçalves ALG, Franco AM, Rossi BA, Gazzoni GAS, Winter ML. Cardiovascular repercussions of the use of SGLT2 inhibitors in patients with type 2 Diabetes Mellitus. Revista Eletrônica Acervo Saúde. 2021 May 18; 13(5):e6890.
- Silva GA, Carreira RG, Garcia WF, Cavasini Filho AE. SGLT2 inhibitors and their influence on the cardiovascular system: a systematic review. Electronic Magazine Acervo Saúde. 2020 Mar 26; (44):e3325.
- The EMPA-KIDNEY Collaborative Group. Empagliflozin in Patients with Chronic Kidney Disease. New England Journal of Medicine. 2022. https://doi.org/10.1056/nejmoa2204233.
- Chertow GM, Vart P, Jongs N, Toto RD, Gorriz JL, Hou FF, McMurray JJV, Correa-Rotter R, Rossing P, Sjöström CD, Stefánsson BV, Langkilde AM, Wheeler DC, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Effects of Dapagliflozin in Stage 4 Chronic Kidney Disease. J Am Soc Nephrol. 2021 Sep;32(9):2352-2361. doi: 10.1681/ASN.2021020167. Epub 2021 Jul 16. PMID: 34272327; PMCID: PMC8729835.
- Waijer SW, Vart P, Cherney DZI, Chertow GM, Jongs N, Langkilde AM, Mann JFE, Mosenzon O, McMurray JJV, Rossing P, Correa-Rotter R, Stefansson BV, Toto RD, Wheeler DC, Heerspink HJL. Effect of dapagliflozin on kidney and cardiovascular outcomes by baseline KDIGO risk categories: a post hoc analysis of the DAPA-CKD trial. Diabetologia. 2022 Jul;65(7):1085-1097. doi: 10.1007/s00125-022-05694-6. Epub 2022 Apr 21. PMID: 35445820; PMCID: PMC9174107.
- Wheeler DC, Stefánsson BV, Jongs N, Chertow GM, Greene T, Hou FF, McMurray JJV, Correa-Rotter R, Rossing P, Toto RD, Sjöström CD, Langkilde AM, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021 Jan;9(1):22-31. doi: 10.1016/S2213-8587(20)30369-7. PMID: 33338413.
- Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Inzucchi SE, Kosiborod MN, Cherney DZI, Dwyer JP, Scirica BM, Bailey CJ, Díaz R, Ray KK, Udell JA, Lopes RD, Lapuerta P, Steg PG; SCORED Investigators. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med. 2021 Jan 14;384(2):129-139. doi: 10.1056/NEJMoa2030186. Epub 2020 Nov 16. PMID: 33200891.
- Bloomgarden Z. The kidney and cardiovascular outcome trials. J Diabetes. 2018 Feb;10(2):88-89. doi: 10.1111/1753-0407.12616. PMID: 29031006.
- Carlos A. Delay in the Progression of Chronic Kidney Disease Using Sglt2 Inhibitors: Integrative Review. Research, Society and Development. 2023; 12: 14. e22212340670-e22212340670. https://doi.org/10.33448/rsd-v12i3.40670.
- Castro RMF. Diabetes Mellitus and Its Complications - A Systematic and Informative Review/ Diabetes Mellitus and Its Complications - a Systematic and Informative Review. Brazilian Journal of Health Review. 2021; 4:3349–3391, www.brazilianjournals.com/index.php/BJHR/article/download/24958/19902, https://doi.org/10.34119/bjhrv4n1-263.
- Fernández-Fernandez B, Sarafidis P, Soler MJ, Ortiz A. EMPA-KIDNEY: expanding the range of kidney protection by SGLT2 inhibitors. Clin Kidney J. 2023 Jun 16;16(8):1187-1198. doi: 10.1093/ckj/sfad082. PMID: 37529652; PMCID: PMC10387399.
- Giugliano D, Longo M, Signoriello S, Maiorino MI, Solerte B, Chiodini P, Esposito K. The effect of DPP-4 inhibitors, GLP-1 receptor agonists and SGLT-2 inhibitors on cardiorenal outcomes: a network meta-analysis of 23 CVOTs. Cardiovasc Diabetol. 2022 Mar 16;21(1):42. doi: 10.1186/s12933-022-01474-z. PMID: 35296336; PMCID: PMC8925229.
- Giusti CT, Carlos PN. Effectiveness and adverse events of Sglt2 Inhibitors. Journal of the Faculty of Medicine of Teresópolis. 4: 2020.
- Hahr AJ, Molitch ME. Management of Diabetes Mellitus in Patients With CKD: Core Curriculum 2022. Am J Kidney Dis. 2022 May;79(5):728-736. doi: 10.1053/j.ajkd.2021.05.023. Epub 2021 Sep 30. PMID: 34600745.
- Handelsman Y. Rationale for the Early Use of Sodium-Glucose Cotransporter-2 Inhibitors in Patients with Type 2 Diabetes. Adv Ther. 2019 Oct;36(10):2567-2586. doi: 10.1007/s12325-019-01054-w. Epub 2019 Aug 23. PMID: 31444707; PMCID: PMC6822830.
- Heerspink HJL, Sjöström CD, Jongs N, Chertow GM, Kosiborod M, Hou FF, McMurray JJV, Rossing P, Correa-Rotter R, Kurlyandskaya R, Stefansson BV, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Effects of dapagliflozin on mortality in patients with chronic kidney disease: a pre-specified analysis from the DAPA-CKD randomized controlled trial. Eur Heart J. 2021 Mar 31;42(13):1216-1227. doi: 10.1093/eurheartj/ehab094. PMID: 33792669; PMCID: PMC8244648.
- Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou FF, Lindberg M, McMurray J, Rossing P, Toto R, Langkilde AM, Wheeler DC; DAPA-CKD Investigators. Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant. 2020 Feb 1;35(2):274-282. doi: 10.1093/ndt/gfz290. PMID: 32030417; PMCID: PMC7005525.
- Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int. 2018 Jul;94(1):26-39. doi: 10.1016/j.kint.2017.12.027. Epub 2018 May 5. PMID: 29735306.
- Jongs N, Greene T, Chertow GM, McMurray JJV, Langkilde AM, Correa-Rotter R, Rossing P, Sjöström CD, Stefansson BV, Toto RD, Wheeler DC, Heerspink HJL; DAPA-CKD Trial Committees and Investigators. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021 Nov;9(11):755-766. doi: 10.1016/S2213-8587(21)00243-6. Epub 2021 Oct 4. PMID: 34619106.
- Kalluri SR, Bhutta TH, Hannoodee H, Al Khalili M, Theik NWY, Raji OE, Shenwai P, Shah R, Khan S. Do SGLT2 Inhibitors Improve Cardio-Renal Outcomes in Patients With Type II Diabetes Mellitus: A Systematic Review. Cureus. 2021 Sep 2;13(9):e17668. doi: 10.7759/cureus.17668. PMID: 34650848; PMCID: PMC8489544.
- Kluger AY, Tecson KM, Lee AY, Lerma EV, Rangaswami J, Lepor NE, Cobble ME, McCullough PA. Class effects of SGLT2 inhibitors on cardiorenal outcomes. Cardiovasc Diabetol. 2019 Aug 5;18(1):99. doi: 10.1186/s12933-019-0903-4. PMID: 31382965; PMCID: PMC6683461.
- Kurata Y, Nangaku M. Dapagliflozin for the treatment of chronic kidney disease. Expert Rev Endocrinol Metab. 2022 Jul;17(4):275-291. doi: 10.1080/17446651.2022.2099373. Epub 2022 Jul 13. PMID: 35822873.
- Lin DS, Lee JK, Hung CS, Chen WJ. The efficacy and safety of novel classes of glucose-lowering drugs for cardiovascular outcomes: a network meta-analysis of randomised clinical trials. Diabetologia. 2021 Dec;64(12):2676-2686. doi: 10.1007/s00125-021-05529-w. Epub 2021 Sep 18. PMID: 34536085.
- McKee A, Al-Khazaali A, Albert SG. Glucagon-like Peptide-1 Receptor Agonists versus Sodium-Glucose Cotransporter Inhibitors for Treatment of T2DM. J Endocr Soc. 2020 Mar 20;4(5):bvaa037. doi: 10.1210/jendso/bvaa037. Erratum in: J Endocr Soc. 2020 Nov 18;4(12):bvaa134. Erratum in: J Endocr Soc. 2021 Jan 21;5(3):bvaa201. PMID: 32342023; PMCID: PMC7182131.
- Mende CW. Chronic Kidney Disease and SGLT2 Inhibitors: A Review of the Evolving Treatment Landscape. Adv Ther. 2022 Jan;39(1):148-164. doi: 10.1007/s12325-021-01994-2. Epub 2021 Nov 30. PMID: 34846711; PMCID: PMC8799531.
- Mendes KDS. Use of bibliographic reference manager in the selection of primary studies in integrative review. Text & Context – Nursing. 2019; 28: www.scielo.br/j/tce/a/HZD4WwnbqL8t7YZpdWSjypj/?lang=pt, https://doi.org/10.1590/1980-265X-TCE-2017-0204.
- Mima A. Sodium-Glucose Cotransporter 2 Inhibitors in Patients with Non-Diabetic Chronic Kidney Disease. Adv Ther. 2021 May;38(5):2201-2212. doi: 10.1007/s12325-021-01735-5. Epub 2021 Apr 16. PMID: 33860925.
- Nunes LC. Association of SGLT2 Cotransporter Inhibitors with the Treatment of Heart Failure: A Literature Review. Electronic Magazine Acervo Médico. 2022; 10: e10336. https://doi.org/10.25248/reamed.e10336.2022.
- Roy A. Kidney Disease in Type 2 Diabetes Mellitus and Benefits of Sodium-Glucose Cotransporter 2 Inhibitors: A Consensus Statement. Diabetes Therapy. 2020; 11: 2791–2827.
- Sarafidis P, Pella E, Kanbay M, Papagianni A. SGLT-2 inhibitors and nephroprotection in patients with diabetic and non-diabetic chronic kidney disease. Current Medicinal Chemistry. 2022 Aug 25; 29.
- Sarafidis P, Ortiz A, Ferro CJ, Halimi JM, Kreutz R, Mallamaci F, Mancia G, Wanner C; ‘Hypertension and the Kidney’ working group of the European Society of Hypertension (ESH) and the ‘European Renal and Cardiovascular Medicine’ (EURECA-m) working group of the European Renal Association - European Dialysis and Transplant Association (ERA-EDTA). Sodium--glucose co-transporter-2 inhibitors for patients with diabetic and nondiabetic chronic kidney disease: a new era has already begun. J Hypertens. 2021 Jun 1;39(6):1090-1097. doi: 10.1097/HJH.0000000000002776. PMID: 33443971.
- Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015 Jan;75(1):33-59. doi: 10.1007/s40265-014-0337-y. PMID: 25488697.
- Kramer CK, Zinman B. Sodium-Glucose Cotransporter-2 (SGLT-2) Inhibitors and the Treatment of Type 2 Diabetes. Annu Rev Med. 2019 Jan 27;70:323-334. doi: 10.1146/annurev-med-042017-094221. Epub 2018 Sep 26. PMID: 30256723.
- Stephens JW, Brown KE, Min T. Chronic kidney disease in type 2 diabetes: Implications for managing glycaemic control, cardiovascular and renal risk. Diabetes Obes Metab. 2020 Apr;22 Suppl 1:32-45. doi: 10.1111/dom.13942. PMID: 32267078.
- Tian L, Ai S, Zheng H, Yang H, Zhou M, Tang J, Liu W, Zhao W, Wang Y. Cardiovascular and renal outcomes with sodium glucose co-transporter 2 inhibitors in patients with type 2 diabetes mellitus: A system review and network meta-analysis. Front Pharmacol. 2022 Nov 24;13:986186. doi: 10.3389/fphar.2022.986186. PMID: 36506550; PMCID: PMC9731650.
- Toyama T, Neuen BL, Jun M, Ohkuma T, Neal B, Jardine MJ, Heerspink HL, Wong MG, Ninomiya T, Wada T, Perkovic V. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab. 2019 May;21(5):1237-1250. doi: 10.1111/dom.13648. Epub 2019 Mar 4. PMID: 30697905.
- Xu B, Li S, Kang B, Zhou J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc Diabetol. 2022 May 25;21(1):83. doi: 10.1186/s12933-022-01512-w. PMID: 35614469; PMCID: PMC9134641.
- Yamada T, Wakabayashi M, Bhalla A, Chopra N, Miyashita H, Mikami T, Ueyama H, Fujisaki T, Saigusa Y, Yamaji T, Azushima K, Urate S, Suzuki T, Abe E, Wakui H, Tamura K. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol. 2021 Jan 7;20(1):14. doi: 10.1186/s12933-020-01197-z. PMID: 33413348; PMCID: PMC7792332.
- Yang Q, Lang Y, Yang W, Yang F, Yang J, Wu Y, Xiao X, Qin C, Zou Y, Zhao Y, Kang D, Liu F. Efficacy and safety of drugs for people with type 2 diabetes mellitus and chronic kidney disease on kidney and cardiovascular outcomes: A systematic review and network meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2023 Apr;198:110592. doi: 10.1016/j.diabres.2023.110592. Epub 2023 Feb 25. PMID: 36842477.
- Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation. 2019 Apr 23;139(17):2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868. PMID: 30786725.
- Zelniker TA, Raz I, Mosenzon O, Dwyer JP, Heerspink HHJL, Cahn A, Goodrich EL, Im K, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Gause-Nilsson I, Langkilde AM, Sabatine MS, Wiviott SD. Effect of Dapagliflozin on Cardiovascular Outcomes According to Baseline Kidney Function and Albuminuria Status in Patients With Type 2 Diabetes: A Prespecified Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol. 2021 Jul 1;6(7):801-810. doi: 10.1001/jamacardio.2021.0660. PMID: 33851953; PMCID: PMC8047725.
- Zhang Q, Zhou S, Liu L. Efficacy and safety evaluation of SGLT2i on blood pressure control in patients with type 2 diabetes and hypertension: a new meta-analysis. Diabetol Metab Syndr. 2023 Jun 7;15(1):118. doi: 10.1186/s13098-023-01092-z. PMID: 37280615; PMCID: PMC10246111.
- Zhang Y, Jiang L, Wang J, Wang T, Chien C, Huang W, Fu X, Xiao Y, Fu Q, Wang S, Zhao J. Network meta-analysis on the effects of finerenone versus SGLT2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus and chronic kidney disease. Cardiovasc Diabetol. 2022 Nov 5;21(1):232. doi: 10.1186/s12933-022-01676-5. PMID: 36335326; PMCID: PMC9637313.
- Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016 Sep 6;134(10):752-72. doi: 10.1161/CIRCULATIONAHA.116.021887. Epub 2016 Jul 28. PMID: 27470878.
- Silva LK, Morais BP, Pinto CT, Maciel JJR, Rezende LG, Moser LL, et al. Developmental mechanisms of euglycemic ketoacidosis associated with the use of SGLT2 inhibitors in patients with Diabetes Mellitus. Brazilian Journal of Health Review [Internet]. 2021 Oct 6 [cited 2023 Mar 24];4(5):21025–38. Available from: https://ojs.brazilianjournals.com.br/ojs/index.php/BJHR/article/view/37014
- Qiu H, Novikov A, Vallon V. Ketosis and diabetic ketoacidosis in response to SGLT2 inhibitors: Basic mechanisms and therapeutic perspectives. Diabetes Metab Res Rev. 2017 Jul;33(5). doi: 10.1002/dmrr.2886. Epub 2017 Feb 23. PMID: 28099783.
- Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI. Sodium Glucose Cotransporter-2 Inhibition in Heart Failure: Potential Mechanisms, Clinical Applications, and Summary of Clinical Trials. Circulation. 2017 Oct 24;136(17):1643-1658. doi: 10.1161/CIRCULATIONAHA.117.030012. PMID: 29061576; PMCID: PMC5846470.