More Information
Submitted: February 26, 2024 | Approved: May 13, 2024 | Published: May 14, 2024
How to cite this article: Asuquo JO, Oyama SE, Samuel SA. The Effect of Variable Doses of Imipramine and Amitriptyline on Learning and Memory. Arch Pharm Pharma Sci. 2024; 8: 047-055.
DOI: 10.29328/journal.apps.1001056
Copyright License: © 2024 Asuquo JO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction inany medium, provided the original work is properly cited.
Keywords: Cognitive memory; Visuospatial learning; Index of discrimination; Amitriptyline; Imipramine; Morris Water Maze (MWM); Novel Object Recognition Task (NORT)
The Effect of Variable Doses of Imipramine and Amitriptyline on Learning and Memory
Asuquo JO1, Oyama SE2, and Seriki A Samuel3*
1Department of Physiology, College of Medical Sciences, University of Calabar, Nigeria
2Department of Family Medicine, University of Alberta, Canada
3Department of Physiology, College of Medical Sciences, Edo State University, Uzairue, Nigeria
*Address for Correspondence: Seriki A Samuel, Department of Physiology, College of Medical Sciences, Edo State University, Uzairue, Nigeria, Email: [email protected]
This study compares the effect of imipramine and amitriptyline on learning and memory. Thirty-five (35) healthy Swiss white (CD1) mice of both sexes weighing 18 g - 30 g were randomly divided into 5 groups (n = 7). Mice in group 1 (control) were administered 0.9% normal saline orally, while mice in groups 2 and 3 were treated with low (1.8 mg/kg) and high (3.7 mg/kg) doses of imipramine, groups 4 and 5 were treated with low (1.8 mg/kg) and high (3.7 mg/kg) of amitriptyline respectively. Treatment was for 21 days before tests. All animals were tested using the Morris Water Maze (MWM) and Novel Object Recognition Task (NORT) to assess visuospatial learning and memory as well as cognitive learning and memory. The results obtained from the Morris Water Maze during the acquisition training showed that the swim latencies were significantly lower (p < 0.05) in the amitriptyline low-dose group compared to the control group. During the reversal training, the swim latencies were significantly lower (p < 0.05) in the test groups compared to the control group. The result for the retention quadrant in the probe trials showed a significant decrease (p < 0.05) in the northeast quadrant in the test groups compared to the control group, with no significant difference in the visible platform day of the Morris Water Maze in the test groups compared to the control group. In the novel object recognition task, the short-term index of habituation was significantly lower (p < 0.05) in the low-dose imipramine and low-dose amitriptyline compared to the control group, the results also showed a significant increase (p < 0.05) in amitriptyline high dose group compared to imipramine and amitriptyline low dose group and the control group. The index of discrimination showed no significant difference among all groups. The long-term index of habituation and discrimination in the memory test showed a significant decrease (p < 0.05) in all the test groups compared to the control group. The results suggest that imipramine and amitriptyline impaired cognitive memory and enhanced visuospatial learning and memory functions.
Chronic medical disorders are often complicated by cognitive impairments with unpleasant consequences, which include disruptions in an academic career, family life, adherence to medical therapy, and managerial abilities. Therefore, medical intervention that can alleviate cognitive disturbance in the affected persons is desirable. Certain drugs such as imipramine and amitriptyline have therefore been reported to enhance different aspects of human life. The human brain is unique in its ability to add to the stock of information; this is described as learning, while the retention and retrieval of this information is memory. So, learning depends on memory [1]. Learning is one of the most important mental functions of humans. It is an adopted change in individual behavior resulting from experience. It relies on the acquisition of different kinds of knowledge supported by perceived information. The mechanism of learning and remembering seems to depend on relatively enduring changes in the nervous system. Its goal is the increase individual and group experience [2].
Learning and memory are closely related; for something to be remembered, it must first be learned. Memory is the faculty of the mind by which information is encoded, stored, and retrieved and it is related to the limbic systems [3]. Often, memory is understood as an informational processing system with explicit and implicit functioning that is made up of sensory processors [4,5].
The word “memory” has three primary definitions first; memory is the location where information is kept in a storehouse or memory store. Second, memory can refer to anything that holds the contents of experience as in a memory trace or engram. Memory is the mental process used to learn, store, or retrieve information of all sorts [6]. In general, memory refers to the storage of information and the processes used to retrieve it. Learning is a term that has a greater association with studies of conditioning that is more likely to involve animals and their environment. Much of the current knowledge of memory has come from studying memory disorders which can result from extensive damage to the regions of the brain including the medial temporary lobe [7] resulting in memory impairment. Some of the memory impairment is a result of a chronic depressive state.
Depression is a prevalent problem with a tendency to relapse and chronicity [8]. According to the World Health Organization Global Burden of Disease Study, unipolar major depression is ranked fourth among all diseases in terms of disability-adjusted life-years and was projected to rank second by the year 2020 [9]. Approximately 15% of the population experiences a major depressive episode at some point in life. Studies have shown that, patients with depression present not only with depressed mood, loss of interest or pleasure, feelings of guilt or low self-esteem, disturbed sleep or appetite, low energy, and poor concentration but also with cognitive deficits [10], among which attention and memory impairment are the most common [11]. Memory impairment observed in patients with depression [12] is usually reversible with effective antidepressant therapy [13]. However, certain antidepressant drugs possess sedating and otherwise impairing adverse effects that can further degrade patients’ functional abilities.
Imipramine and amitriptyline belong to a class of drugs known as Tricyclic Antidepressants (TCA) that are being used to treat anxiety and depressive states. Prolonged use of this drug causes tolerance and may lead to both physical and psychological dependence on the drug [14]. This drug is reported to be metabolized by a hepatic enzyme belonging to the cytochrome P450 family of enzymes [15]. Their half-life which ranges from 10 - 50 hours with plasma concentration occurring approximately 1 hour after its oral administration, has been used in the pharmacological treatment of anxiety since the early 60’s. It is used therapeutically to produce sedation, induce sleep, relieve anxiety and muscle spasms, and prevent seizures. Their mechanism of action on the Central Nervous System is believed to be related to their ability to increase serotonin and norepinephrine reuptake in the brain and downstream the effect of Gamma Amino Butyric Acid (GABA) which is a major inhibitory neurotransmitter in the brain [16-19].
Apparatus: Morris water maze, novel object recognition task, force swim test, and nesting test.
Experimental animals
Thirty-five (35) albino mice of different sexes weighing about 18 g – 28 g were used for this study. The mice were purchased from the Department of Physiology, University of Calabar, Calabar, and were kept under standard conditions in the animal house, Department of Physiology, University of Calabar, and given free access to rodent chow and water. The animals were allowed to acclimatize under standard conditions in 12-hour light/dark cycles for 7 days before the experiment began.
Experimental design: The animals were randomly selected and assigned into five (5) groups of seven mice each (n = 7).
Group one: (control group)- mice in this group were administered with 0.9% normal saline.
Group two: (imipramine low-dose group)- mice in this group were treated with 1.8 mg/kg bwt.
Group three: (imipramine high-dose group)- mice in this group were treated with 3.7 mg/kg bwt.
Group four: (amitriptyline low-dose group)- mice in this group were treated with 1.8 mg/kg bwt.
Group five: (amitriptyline high-dose group)- mice in this group were treated with 3.7 mg/kg bwt.
Conducting research
The search was carried out using the Pubmed, Embase, and Virtual Health Library databases, with the guiding question being “What are the benefits of using SLGT2 inhibitors for patients with chronic kidney disease and type 2 diabetes mellitus?”.
The database was searched using the following Health Science Descriptors (DeCS): “SLGT-2 inhibitor”, “Sodium-glucose Cotransporter-2 Inhibitors”, “Type 2 diabetes”, “Chronic Kidney disease”, “Death”, using the Boolean operators “AND” and “OR”. Thus, the search strategy used in all the databases was: (“SLGT-2 inhibitor” OR “canagliflozin” OR “dapagliflozin” OR “empagliflozin” OR “Sodium-glucose Cotransporter-2 Inhibitors”) AND (“DM2” OR “type 2 diabetes”) AND (“chronic kidney disease”) AND(“death” OR “mortality”).
Drug collection and preparation
Amitriptyline, normal saline, and imipramine were purchased from Bez Pharmaceuticals. All drugs were prepared by dissolving in 0.9% normal saline and were administered orally at an administration rate of 0.1 ml/10g body weight. 25 mg of Imipramine and amitriptyline was dissolved in 13.4 ml and 6.7 ml of normal saline to constitute a dose of 3.7 mg/ml and 1.8 mg/ml for low and high doses of imipramine and amitriptyline respectively. All the animals were treated for 21 days before commencing behavioral studies and a one-day interval between studies.
Behavioural assay
Cognitive memory function and visuo-spartial memory and learning for all the animals were assessed using the Novel Object Recognition Task [20] and the Morris Water Maze Test [21].
Morris water maze
The Morris Water Maze (MWM) consists of a circular polypropylene pool that measures 110 cm in diameter and 20cm in depth filled with opaque water. Rats or mice are trained to use extra-maze visual cues to locate an extra platform hidden just below the surface of the opaque water [21]. The hidden platform version of the MWM is a test of visuospatial learning and memory which is impaired by hippocampal lesions [22]. The MWM that was used for this study was modified for mice [23].
The pool was filled to a depth of 14 cm (0.5 cm over the escape platform) with room-temperature tap water which is made opaque with the addition of ground non-toxic chalk. The water was left to sit overnight to reach room temperature (22+/- oc).
The pool is divided into four quadrants: Northwest, Northeast, Southwest, and Southeast. Boundaries of these quadrants were marked on the edges of the pool with masking tape and labeled: North, South, East, and West. An escape platform of a Plexiglas cylinder (13.5 cm × 9 cm diameter) filled with cement to make firm was suspended and hidden 0.5 cm beneath the pool.
The MWM is an experimental test protocol that lasted for 8 days as follows:
Day 1: Acquisition Day 1
Day 2: Acquisition Day 2
Day 3: Acquisition Day 3
Day 4: Reversal Day 1
Day 5: Reversal Day 2
Day 6: Reversal Day 3
Day 7: Probe trial
Day 8: Visible platform day
Acquisition and reversal training were done with the escape platform hidden 0.5 cm below the opaque water; in the North-East quadrant during acquisition training and in the South-West quadrant during reversal training. During the probe trial, there is no escape platform so that visuo-spatial memory can be assessed. On the visible day, the platform is to another quadrant (Northwest) of the pool, and the visible top is added to the platform. This assesses basic visual ability and motivation to locate the platform.
During the acquisition training (Day 1-3), the platform was placed (and hidden 0.5 cm below) in the centre of the Northeast quadrant. Each mouse was given a maximum of 60 seconds to locate the hidden platform within the allotted time, it was then allowed at least 10 seconds on the platform to view extra maze cues after which it was removed from the pool using a small container, and the swim latency (i.e. the time it takes the animal to locate and climb the escape platform was recorded.
It is important to only remove the mouse after it is on the platform so that it associates the platform with escape. If the mouse does not find the platform during the allotted time, the animal is guided onto the platform using the plastic container and allowed for 10 seconds before it is taken out of the pool. When the animal is removed from the pool, it is usually kept in a holding cage where their body is dried using a paper towel before being returned to their home cages. The next mouse is then placed in the pool and the same procedure is followed. Each animal completed 4 trials per day over 3 days, for 12 trials of acquisition training, each trial from a different one of the 4 start locations.
Reversal training begins on day 4 and ends on day 6. The location of the escape platform was changed to the opposite quadrant (Southwest quadrant), and mice were again assigned to appropriate start positions. The same procedures as in acquisition training were carried out during reversal training. Each of the animals completed 4 trials per day for 3 days for a total of 12 trials of reversal training.
A probe trial was conducted on day 7 to assess visuo-spatial memory. On that day, there was no escape platform in the maze. Each mouse was placed in the pool from one of the four possible start positions (North Pole) and allowed to explore the pool for 60 seconds, during which the time spent in each quadrant of the maze was recorded. When the 60-sec was complete the mouse was scooped up using the container and placed in a holding cage to dry before being returned to its home cage. It is believed that animals with good visuo-spatial memory would spend more time in the quadrants where the escape platform was located.
The visible platform task was conducted on day 8. The visible platform was placed in a new location within the Northwest quadrant of the pool but this time made visible through the attachment of a colourful detachable flag to the top of the platform. The same procedures as in acquisition and reversal training were carried out and the mice completed 4 trials.
Novel Object Recognition Task (NORT)
The Novel Object Recognition Task (NORT) was originally developed for rats as a test of declarative memory after it was discovered that rats will spend more time investigating a new object than a familiar one [20]. It has since been validated as a test of recognition memory in mice [24-26].
The object recognition task is conducted in an open field (OF) box (38 x 38 cm), which is also used to assess exploratory behaviour. The floor and three walls are made from 2 cm thick plywood that has been painted white. The fourth wall is made of clear Plexiglas so that the mice can be observed from the front of the apparatus as well as from the top. Blue lines painted on the floor divide the open field into forty-nine 5 x 5 cm squares, and these lines are used to assess locomotor activity. The centre square (15 x 15 cm) is formed from the four inner squares and this square is highlighted with a black marker. A sheet of clear Plexiglas covers the floor.
Before testing all mice are habituated to the apparatus for 5-min 24 hours beforehand. Mice were carried to the test room in their home cages and run individually.
Procedure: Mice were moved from their home cage to the testing apparatus and back using a small container. After each 5-minute trial, the mice were returned to their home cages and the apparatus was cleaned with 70% ethyl alcohol and permitted to dry between trials.
There are two different ways of conducting the novel object test, each of which focuses on a different type of memory depending on the retention period between training the objects (trial 1) and testing (trial 2). Two pairs of identical objects are needed. The first method involves a short duration between the introduction of the novel objects and testing. Both trials (acquisition and recognition) are on the same day, separated by a retention period of 5-min, 15-min, 30-min or 1-h. During the first trial, two identical objects (O1 and O2) are placed in diagonal corners opposite each other in the open field. Objects are secured to the floor of the apparatus with reusable adhesive (Tac’NStik, Ross Products, Toronto, ON). The mice were scooped up from their home cage in a small plastic cup and placed in the middle of the open field arena. Each mouse is allowed to explore the arena and objects for 5-min. At the end of the trial, the mouse is removed from the apparatus using the plastic cup and returned to its home cage. After a 15-min, 30-min, or 1-h inter-trial interval (retention period) the mouse is returned to the test apparatus (trial 2). The arena now contains the familiar object (Q1 or Q2 from trial 1) in one of the two locations in trial 1 and a new object (N) that replaces Q1 or Q2. The same behaviours recorded for trial 1 are recorded for 5-min for trial 2.
The second type of novel object recognition task, which is a test of long-term memory, involves 24 hours between trial 1 and trial 2. All other methodology remains the same.
Metal and plastic objects of various shapes ranging in size from about 2 x 2 x 2 cm to 2 x 4 x 6 cm (jar lids, bolts, nuts) were used as objects. The object to be replaced in trial 2 was counterbalanced for each group of animals, as was which pair of objects was used in trial 1. The location of the familiar object and novel object was also counterbalanced for each group. The new object (N) was similar in size to Q1 to reduce preference for either object. All objects and the apparatus were cleaned using 70 % ethyl alcohol to eliminate olfactory stimuli.
Statistical analysis
Data obtained from the study will be expressed as mean ± SEM following one-way analysis of variance (ANOVA) and statistical comparison among the groups will be performed with Turkey multiple comparison test using SPSS, version 17. 0. p < 0.05 will be considered statistically significant.
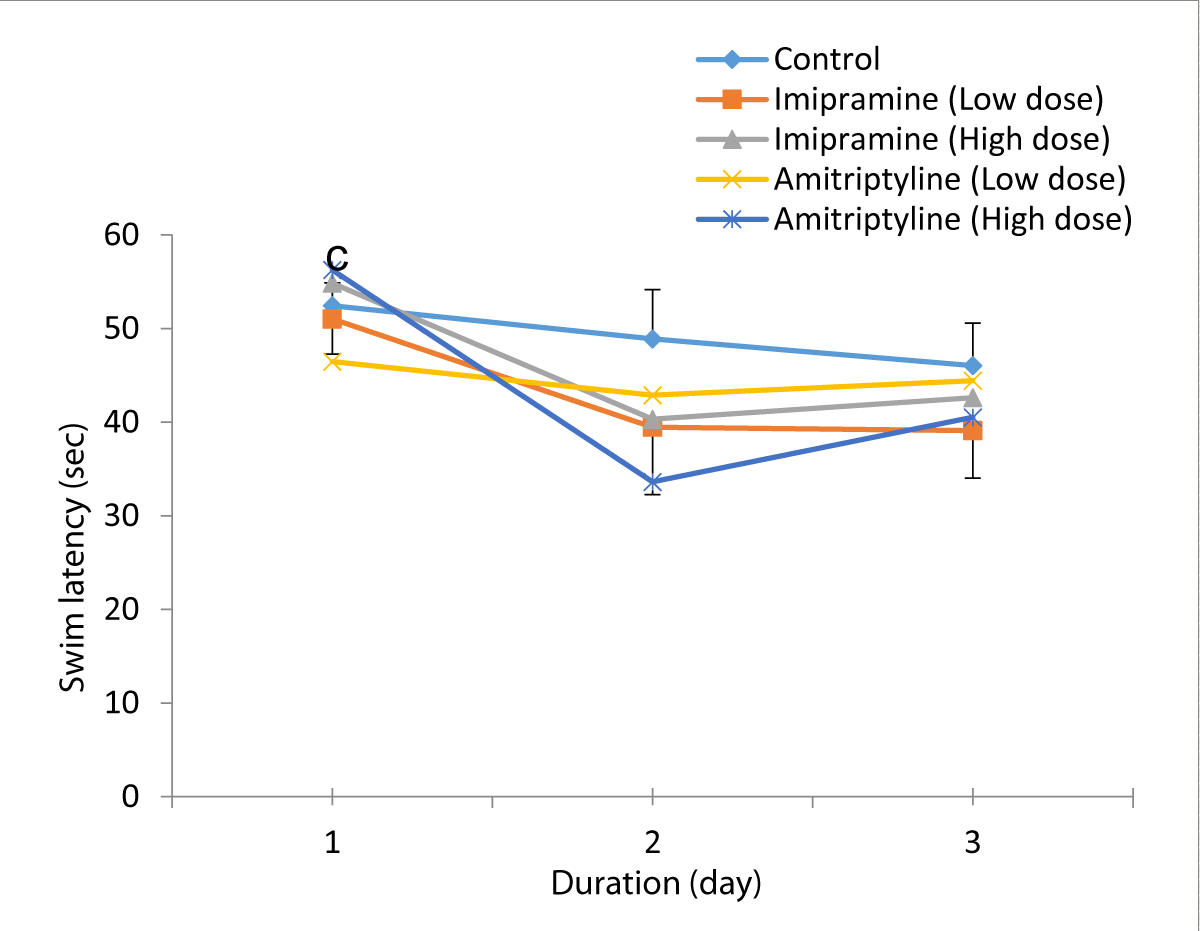
The comparison of swim latency in the test groups and control groups during the acquisition training periods of the Morris Water Maze test is shown in Figure 1.
Figure 2: Comparison of swim latency in the different experimental groups during the acquisition training periods of the Morris water maze test. Values are expressed as mean + SEM, n = 6. c = p < 0.05 vs. Amitriptyline (LD).
The mean for day 1, were 52.45 ± 2.43, 51.02 ± 3.74, 54.85 ± 2.31, 46.46 ± 4.90, and 56.26 ± 2.48 for control, low dose imipramine, high dose imipramine, low dose amitriptyline, and high dose amitriptyline respectively. The result showed that there was a significant increase (p < 0.05) in imipramine low dose, high dose groups, and amitriptyline high dose group when compared to the amitriptyline low dose group.
Acquisition training day 2, the mean ± SEM were: 48.89 ± 5.26, 39.47 ± 7.21, 40.32 ± 7.32, 42.89 ± 5.49, and 33.63 ± 5.63 for control, low dose imipramine, high dose imipramine, low dose amitriptyline and high dose amitriptyline respectively. The result showed that there was a significant increase
(p < 0.05) in the imipramine low-dose, high-dose groups, and amitriptyline high-dose group compared to the amitriptyline low-dose group and the control group.
On acquisition day 3. The mean ± SEM swim latency for day 3 were: 46.05 ± 4.54, 39.11 ± 5.08, 42.63 ± 8.36, 44.43 ± 5.20, 40.52 ± 7.77 for control, low dose imipramine, high dose imipramine, low dose amitriptyline and high dose amitriptyline respectively. The result also showed a similar trend as seen in day 2.
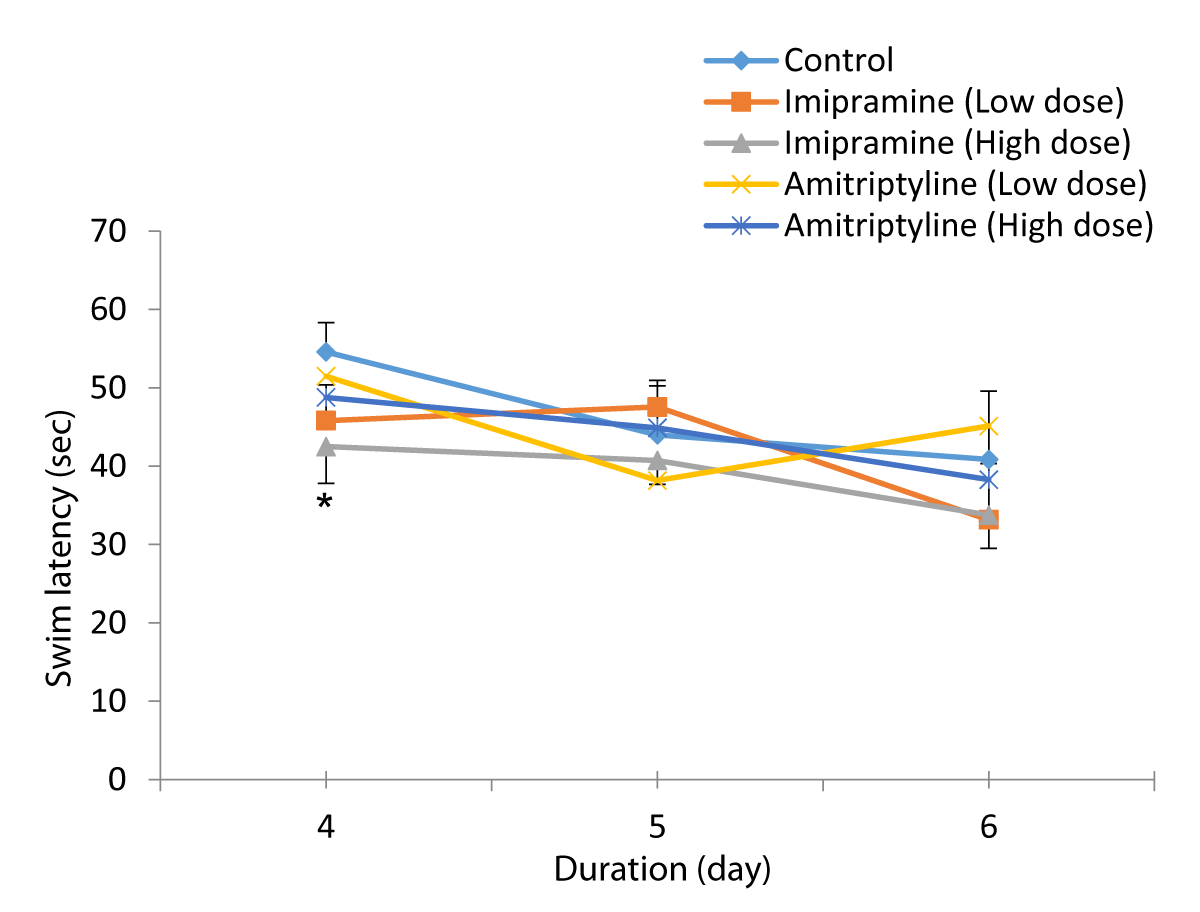
The comparison of swim latency in test groups and control group during reversal training periods of the Morris water maze test is shown in Figure 2. Reversal training period day 1, the mean ±SEM swim latency for day 1 were: 54.57± 3.75, 45.81 ± 4.57, 42.49 ± 4.69, 51.48 ± 2.67, and 48.77 ± 3.56 for Control, low dose imipramine, high dose imipramine, low dose amitriptyline and high dose amitriptyline respectively. The result showed that there was a significant decrease at (p < 0.05) in all the test groups compared to the control group.
Figure 2: Comparison of swim latency in the different experimental groups during the reversal training periods of the Morris water maze test. Values are expressed as mean + SEM, n = 6. * = p < 0.05 vs. control.
Reversal training period day 2, swim latency mean ± SEM for day 2 were: 43.99 ± 6.97, 47.55 ± 2.69, 40.72 ± 3.04, 38.17 ± 5.18 and 44.87 ± 3.68 for Control, low dose imipramine, high dose imipramine, low dose amitriptyline and high dose amitriptyline respectively. The result showed a significant increase (p < 0.05) in the low-dose imipramine compared to the control group.
Reversal training period day 3, swim latency mean ± SEM for day 3 were: 40.85 ± 8.74, 33.15 ± 7.17, 33.76 ± 4.76, 45.13 ± 4.61, and 38.28 ± 6.62 for control, low dose imipramine, high dose imipramine, low dose amitriptyline and high dose amitriptyline respectively. The result showed a significant decrease (p < 0.05) in the amitriptyline low-dose group compared to the control group.
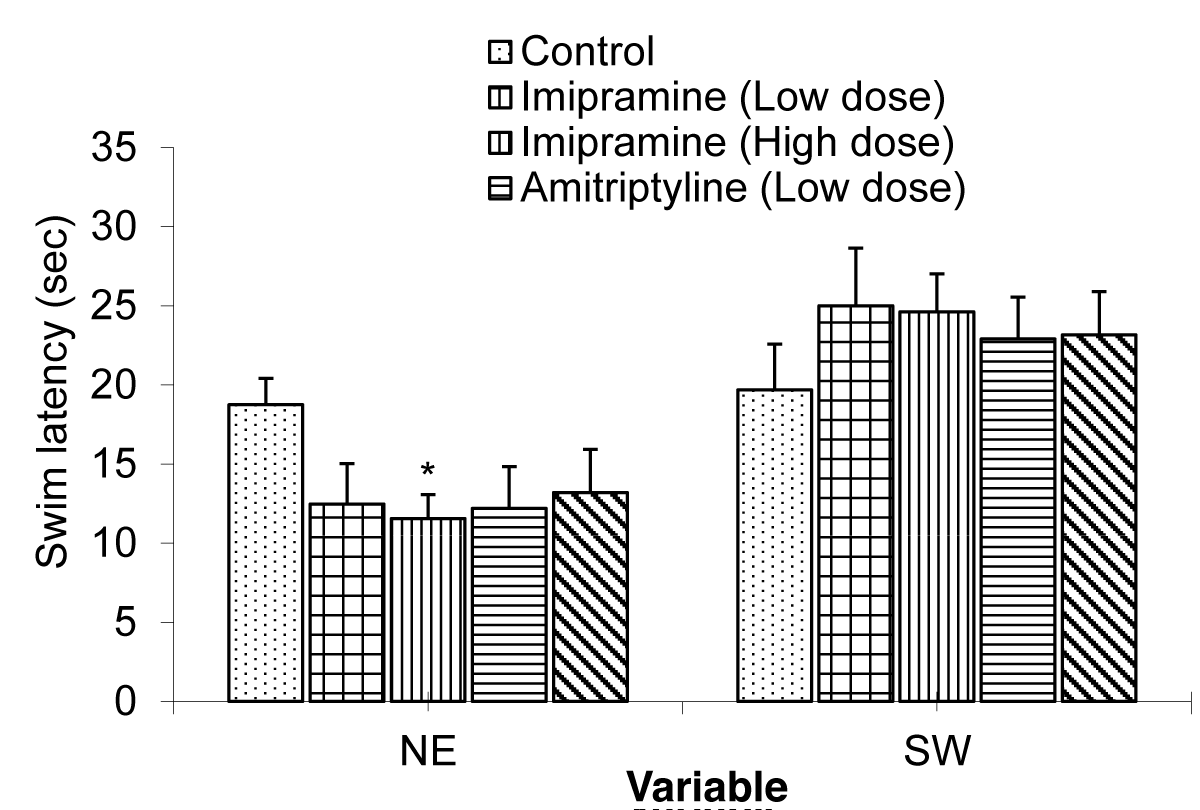
The Comparison of quadrant duration in the test groups and control group during the probe trial (day 7) of the Morris water maze test is shown in Figure 3.
Figure 3: Comparison of annulus acquisition and reversal crossing during the Morris water maze task treated with low and high doses of imipramine and amitriptyline. Values are expressed as mean + SEM, n = 6.
The mean ± SEM of quadrant duration for NE were: 18.75 ± 1.66, 12.49 ± 2.56, 11.55 ± 1.51, 12.21 ± 3.77, and 13.20 ± 1.00 Control, low dose imipramine, high dose imipramine, low dose amitriptyline and high dose amitriptyline respectively.
The result showed that there was a significant decrease in the imipramine high dose at (p < 0.05) when compared to the control group and other test groups.
The mean ± SEM of quadrant duration for SW were: 19.69 ± 2.89, 25.01 ± 3.64, 24.62 ± 2.4, 22.91 ± 2.37, and 23.16 ± 5.88 for control, low dose imipramine, high dose imipramine, low dose amitriptyline and high dose amitriptyline respectively. The result showed that there was no significant difference between the test groups when compared to the control group.
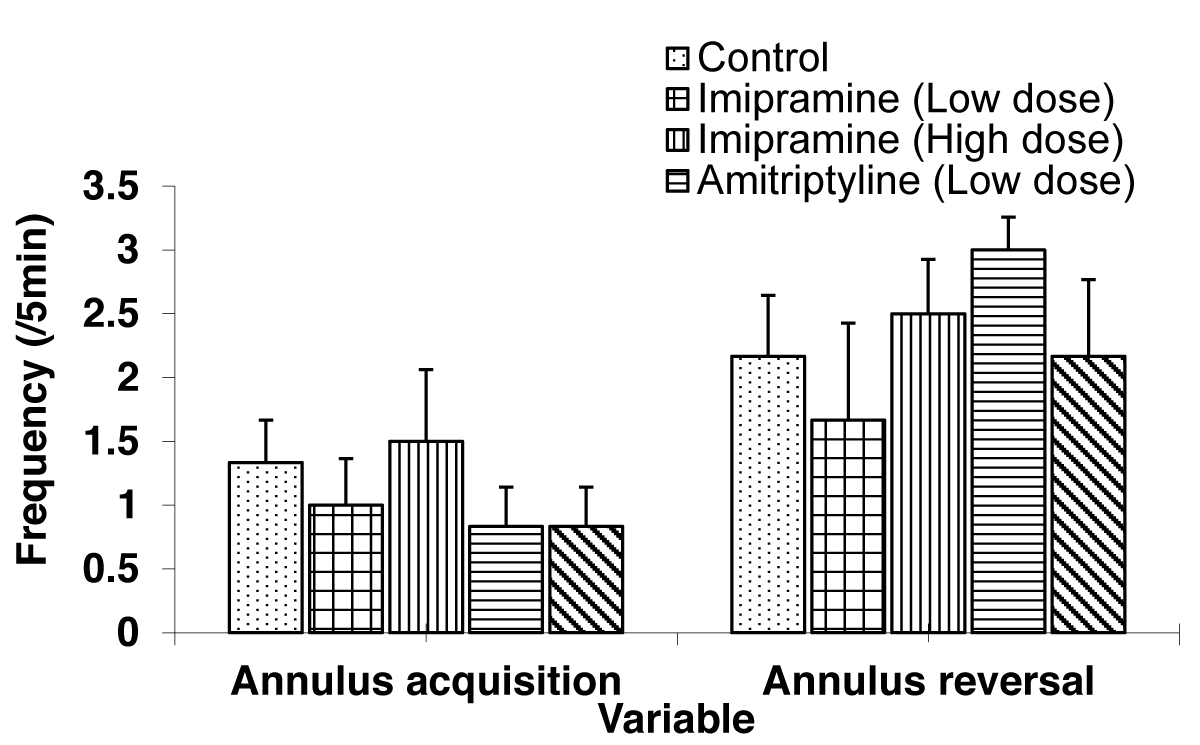
The Comparison of annulus acquisition and reversal crossing in the test groups and control group during the probe trial (day 7) of the Morris water maze test is shown in Figure 4.
Figure 4: Comparison of annulus acquisition and reversal crossing during the Morris water maze task treated with low and high doses of imipramine and amitriptyline. Values are expressed as mean + SEM, n = 6.
The mean ± SEM of annulus acquisition crossing were: 1.33 ± 0.33, 1.00 ± 0.37, 1.50 ± 0.56, 0.83 ± 0.31, and 0.83 ± 0.31 for control, low dose imipramine, high dose imipramine, low dose amitriptyline and high dose amitriptyline respectively. The result showed that there was no significant difference in the test groups when compared to the control group.
The mean ± SEM of annulus reversal crossing were: 2.17 ± 0.48, 1.67 ± 0.76, 2.50 ± 0.43, 3.00 ± 0.26 and 2.17 ± 0.60 for control, low dose imipramine, high dose imipramine, low dose amitriptyline, and high dose amitriptyline respectively. The result showed no significant difference in the test groups compared to the control group.
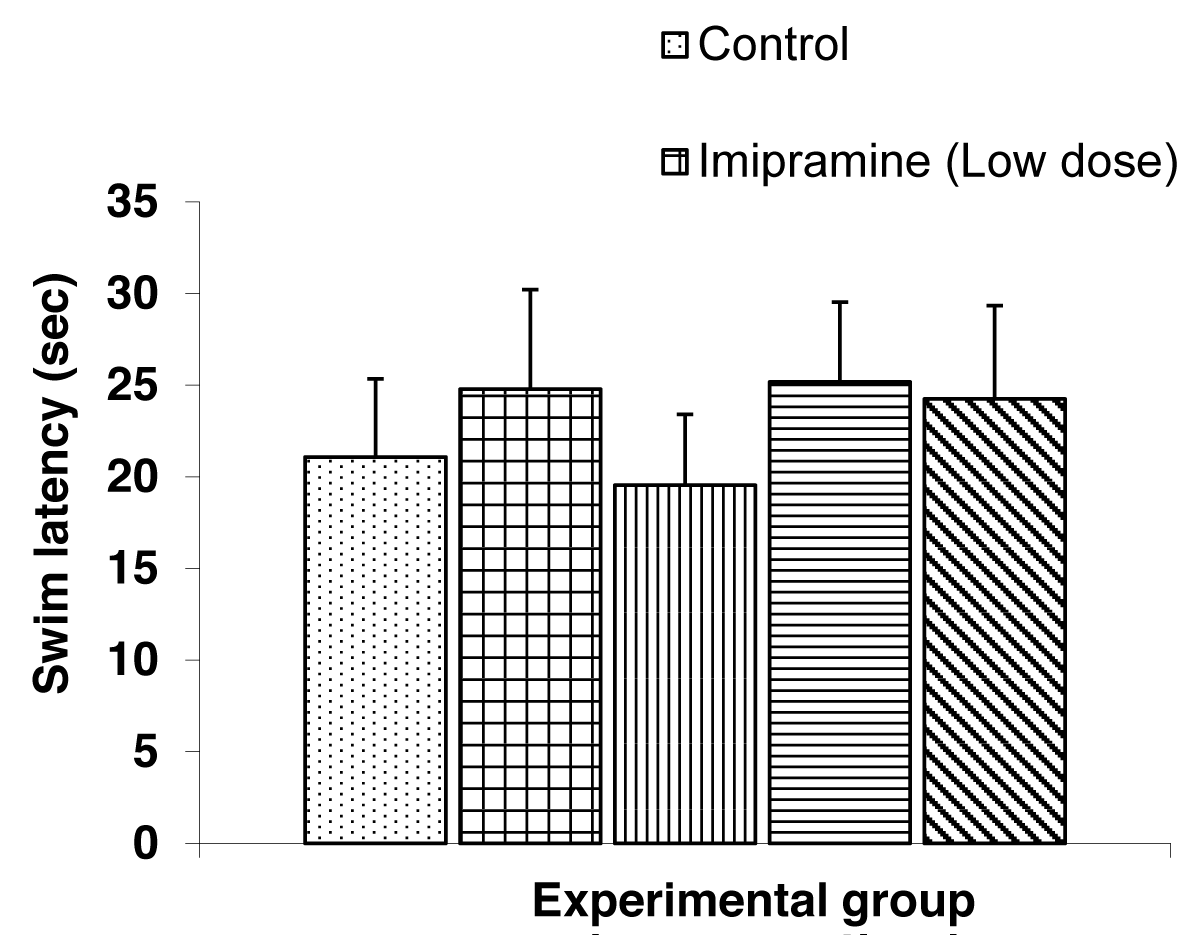
The Comparison of swim latency in the test groups and control group during the visible platform (day 8) of the Morris water maze test is shown in Figure 5.
Figure 5: Comparison of Swim latencies during the visible platform of the Morris water maze tasks treated with low and high doses of imipramine and amitriptyline. Values are expressed as mean + SEM, n = 6.
The mean ± SEM swim latency during the visible platform day were, 21.07 ± 4.27, 24.78 ± 5.43, 19.53 ± 3.86, 25.17 ± 4.36, and 24.24 ± 5.10 for control, low dose imipramine, high dose imipramine, low dose amitriptyline and high dose amitriptyline respectively. The result showed no significant difference between the test groups and the control group.
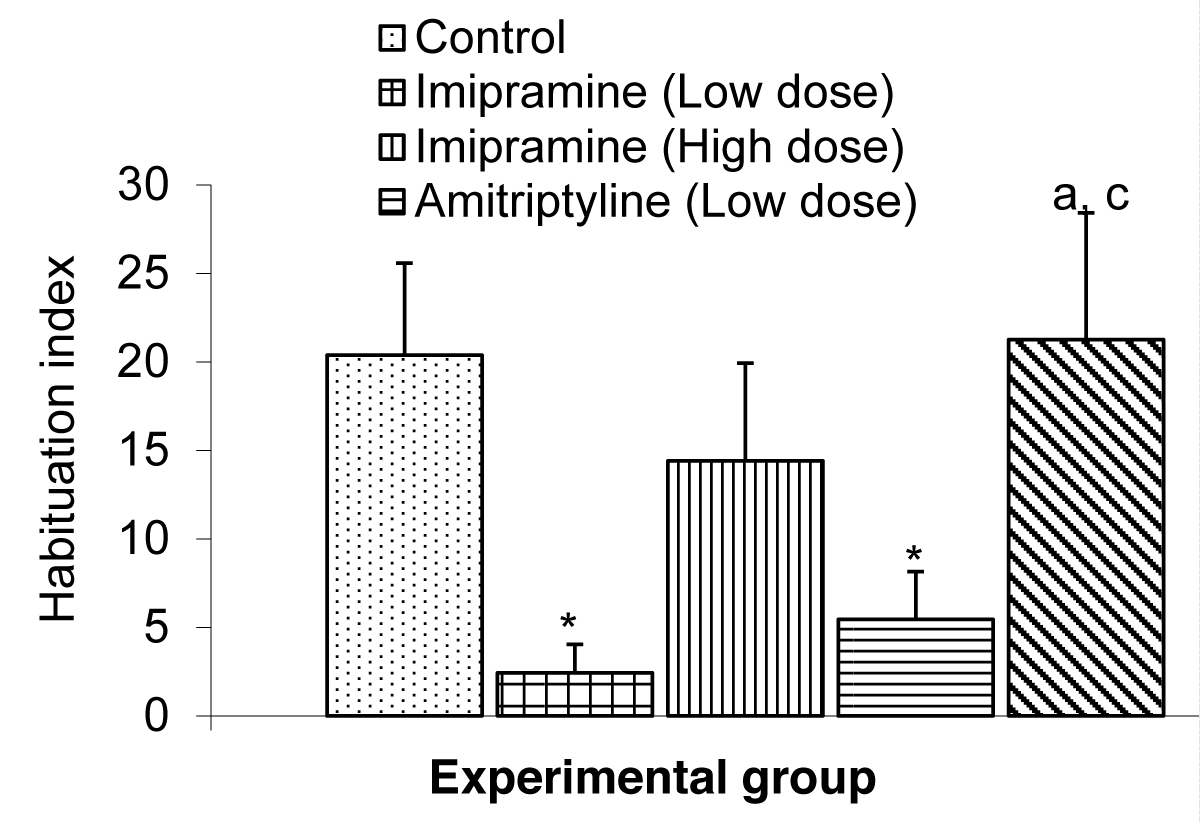
The Comparison of the index of habituation in the short-term memory test during the NORT in mice is shown in Figure 6.
Figure 6: Comparison of habituation index in the short term memory during the NORT test in mice treated with low and high doses of imipramine and amitriptyline. Values are expressed as mean + SEM, n = 6. * = p < 0.05 vs. control; a = p < 0.05 vs. imipramine.
The mean ± SEMs for the index of habituation were 20.37 ± 5.19, 2.43 ± 1.62, 14.41 ± 5.52, 5.45 ± 2.71, and 21.26 ± 7.15 for the control low dose imipramine, high dose imipramine, low dose amitriptyline and high dose amitriptyline respectively. The result showed that there was a significant decrease (p < 0.05) in the low-dose imipramine and low-dose amitriptyline compared to the control group. However, the result also showed a significant increase (p < 0.05) in the amitriptyline high-dose group compared to the imipramine and amitriptyline low-dose group.
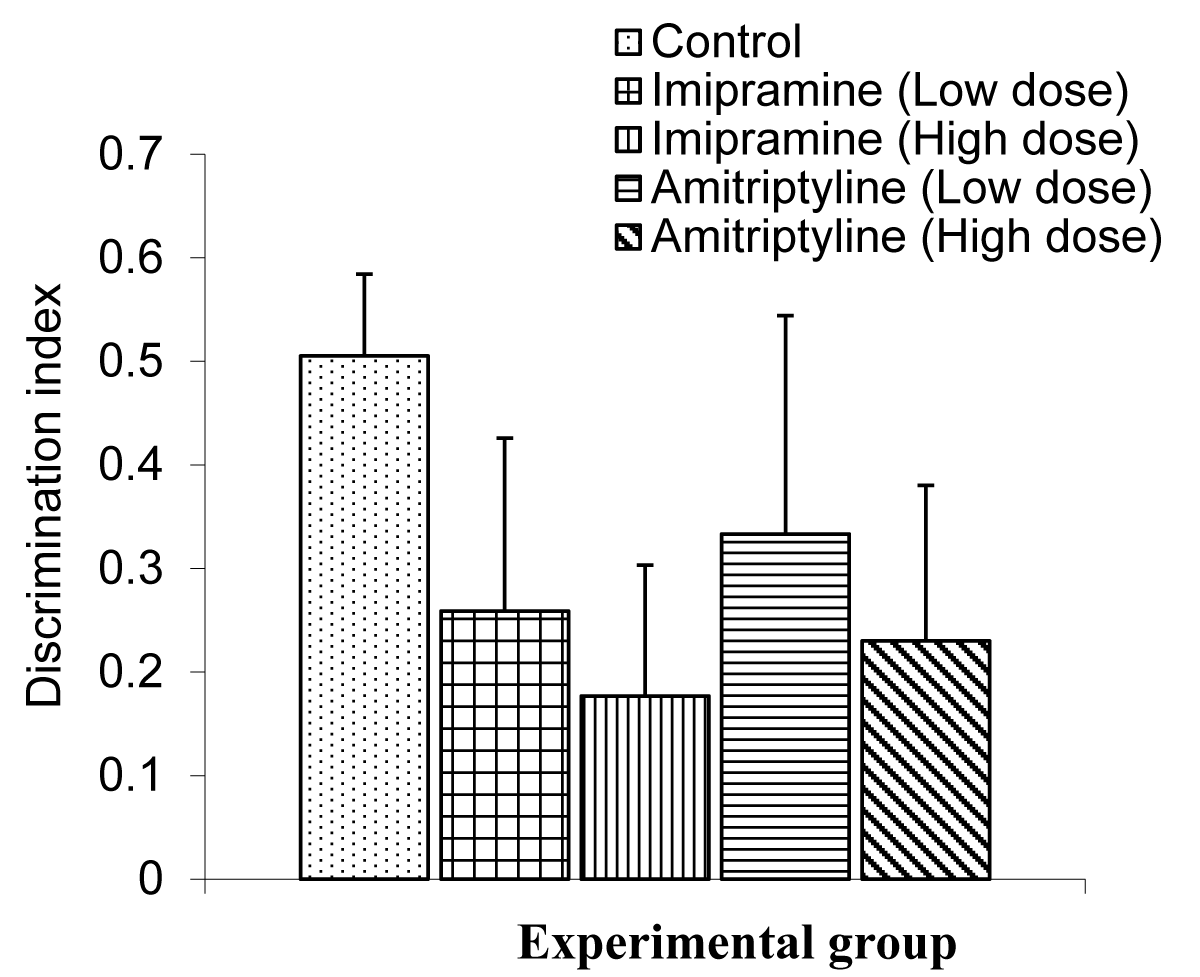
The Comparison of index discrimination in the short-term memory test during the NORT in mice is shown in Figure 7.
Figure 7: Comparison of index of discriminiation in the short term memory during NORT test in mice treated with low and high doses of imipramine and amitriptyline. Values are expressed as mean + SEM, n = 6.
The mean ± SEMs for the index of discrimination were 0.51 ± 0.08, 0.26 ± 0.17, 0.18 ± 0.13, 0.33 ± 0.21 for the control, low dose imipramine, high dose imipramine, low dose amitriptyline, and high dose amitriptyline respectively.
The result showed that there was no significant difference between the test groups and the control group.
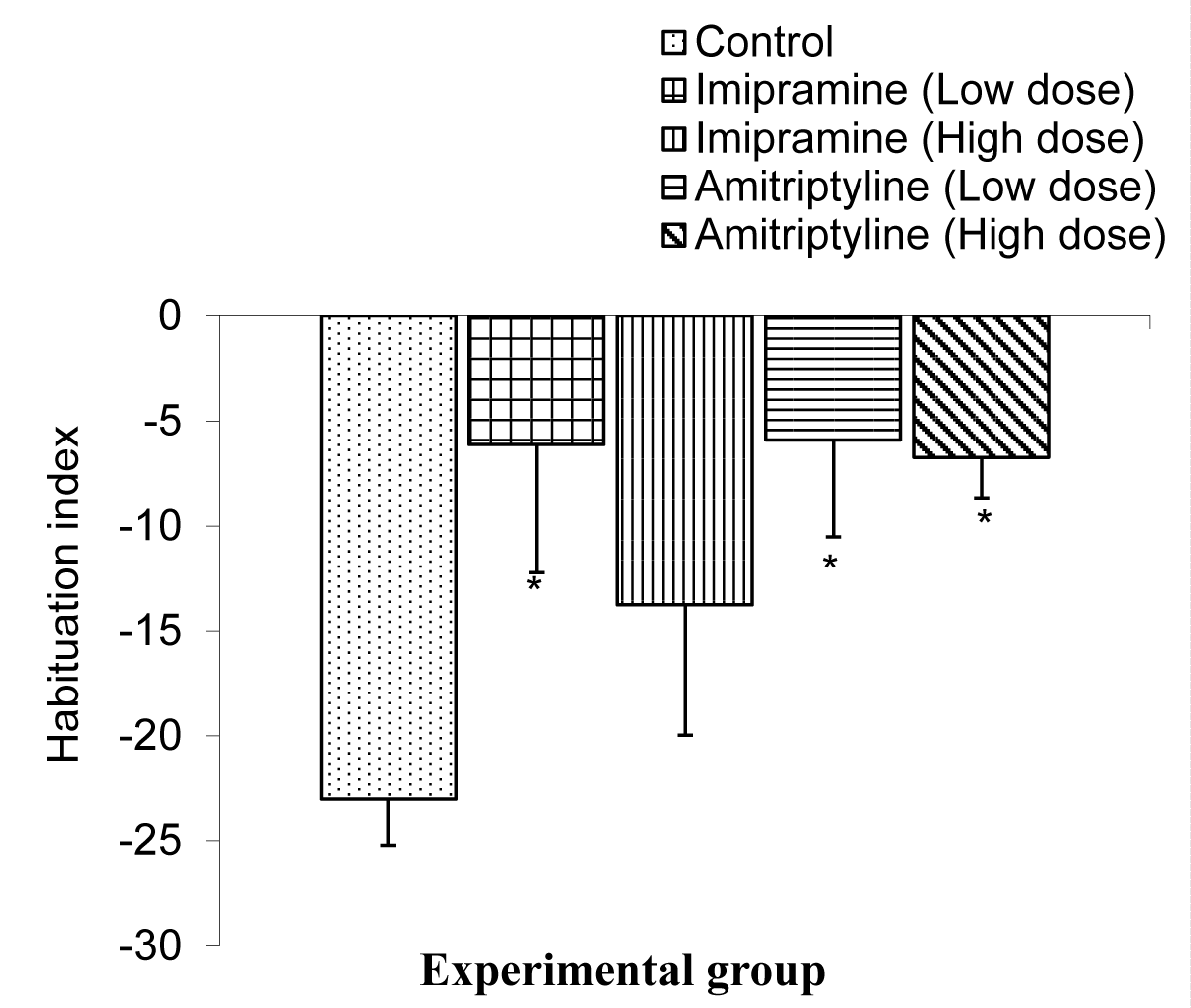
The Comparison of the index of habituation in the long-term memory test during the NORT in mice is shown in Figure 8.
Figure 8: Comparison of index of habituation in the long term memory during the NORT test in mice treated with low and high doses of imipramine and amitriptyline. Values are expressed as mean + SEM, n = 6. * = p < 0.05 vs. control.
The mean ± SEMs for the index of habituation were -22.98 ± 2.25, -6.12 ± 6.10, -13.76 ± 6.62, -5.90 ± 4.61, and -6.73 ± 1.93 for the control, low dose imipramine, high dose imipramine, low dose amitriptyline, and high dose amitriptyline respectively. The result showed that there is a significant decrease (p < 0.05) in the imipramine low dose, amitriptyline low dose, and high dose group when compared to the control group.
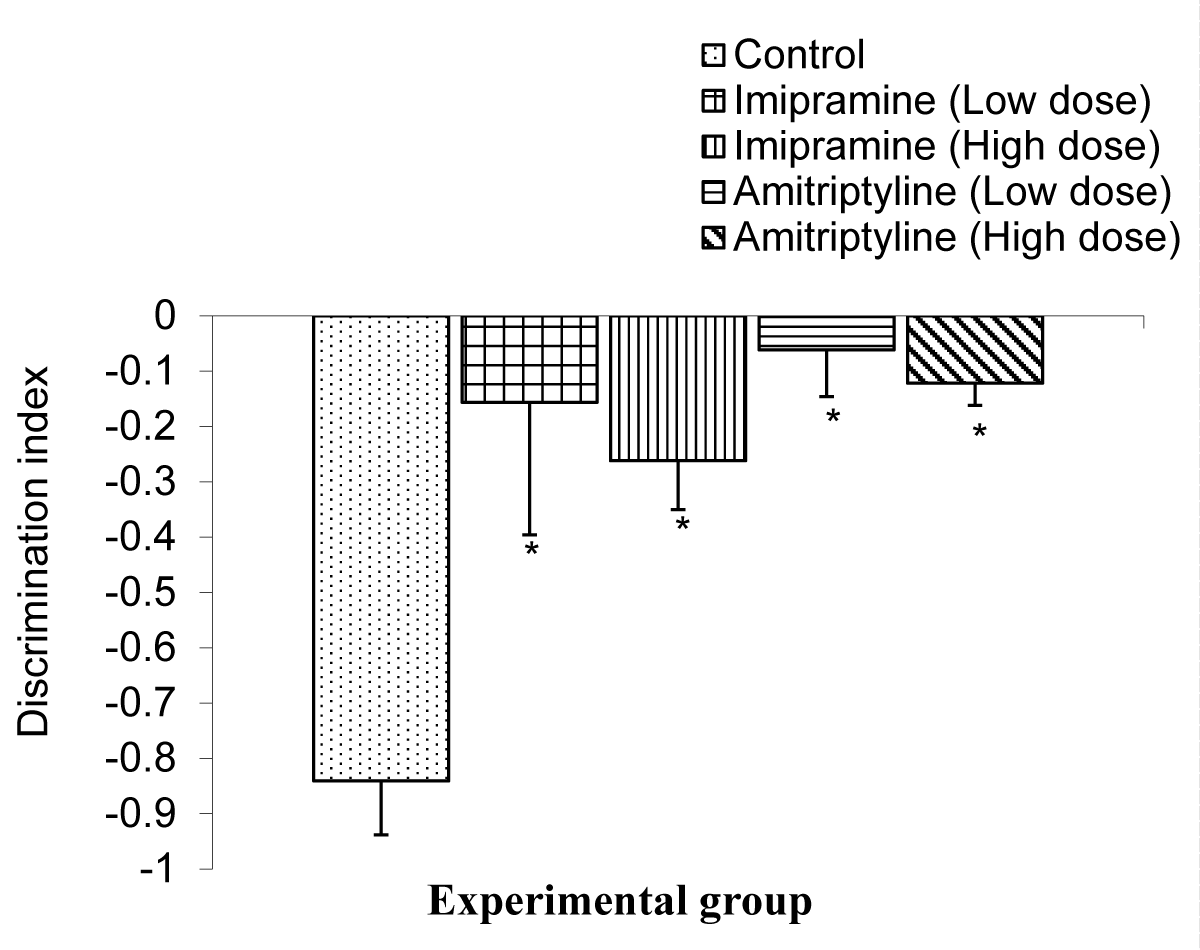
The Comparison of the index of discrimination in long-term memory tests during the NORT in mice is shown in Figure 9.
Figure 9: Comparison of index of discriminiation in the long term memory during the NORT test in mice following treatment low and high doses of imipramine and amitriptyline.Values are expressed as mean + SEM, n = 6. * = p < 0.05 vs. control
The mean ± SEMs for index of discrimination were -0.84 ± 0.10, - 0.16 ± 0.24, - 0.26 ± 0.09, -0.06 ± 0.08, and -0.12 ± 0.04 for the control, low dose imipramine, high dose imipramine, low dose amitriptyline, and high dose amitriptyline respectively. The result showed that there was a significant decrease (p < 0.05) in all the test groups when compared to the control group.
The hidden platform version of the Morris Water Maze is a test of visuospatial learning and memory performance which is impaired by hippocampal lesion [22] the visible-platform (cued) version of the Morris Water Maze, on the other hand, is a non-hippocampal task, which is dependent on the dorsal straintum (caudate nucleus and putamen) of the basal ganglia [22].
The visible (cued) platform uses a unique intra-maze visual/cue that is placed at the location of the escape platform whereas the vision-spatial learning task uses extra-maze cues.
The swim latency is defined as the time it took the mice to locate the hidden platform in the visuospatial learning task or the cued platform in the visible platform task. The shorter the swim latencies, the better the learning processes. Mice who learned faster were able to figure out the spatial location/position of the hidden platform earlier than their counterparts. Also, the steeper the gradient of swim latency within the three-day acquisition or reversal training the better the learning curve, hence learning.
Following administration of low and high doses of imipramine and amitriptyline, there was a significant decrease between the swim latencies during the acquisition trial in mice administered low dosage of amitriptyline compared to the amitriptyline high dose, imipramine low dose and high dose and control group. This means that mice in the group treated with low-dose amitriptyline were able to find the hidden platform faster than their counterpart groups. However, following the trend of results from days 2 and 3, imipramine and amitriptyline therefore did not affect learning during acquisition training.
During reversal training, the swim latencies for the groups of mice administered low to high doses of imipramine (1.8 and 3.7 mg/kg, orally) showed a significant decrease when compared to the amitriptyline low and high doses and the control group.
Visuospatial memory was assessed during the probe trial in the Morris Water Maze task. During the probe trial day, the hidden platform was removed and mice were introduced into the maze and allowed to explore for 60 seconds, during which the quadrants’ duration (the time spent exploring each quadrant) were recorded. It is expected that mice that have a good memory of the spatial location/position of the hidden platform would spend more time exploring the quadrant that had the platform during the reversal training, in this case, the Southwest (SW) quadrant. Mice treated with (3.7 mg/kg) high dose imipramine showed a significant decrease in the time spent in the SW quadrant when compared to the control group.
This implies that administration of high doses of imipramine impairs memory. The cued version of Morris Water Maze assesses the cued learning and visual integrity of the animals tested. Impairment in performance in the hidden-platform task may be due to some brain lesions or drugs which may have affected the motivation to escape. For instance, neurons in the different regions of the brain comprising the reward system communicate using dopamine; for example, dopamine-producing neurons in the brain’s ventral tegmental area communicate with those in a region called the nucleus accumbens to process rewards and motivate behaviour. Neurons that release dopamine are activated when we expect to receive a reward. Dopamine also enhances reward-related memories. It strengthens synapses, the junctions where neurons pass messages in the brain’s learning and memory center, the hippocampus. Dopamine signaling in areas of the brain that process emotions the amygdala and regions involved in planning and reasoning the prefrontal cortex also creates emotional associations with rewards. Learning and motivation are strongly influenced by the hippocampus and amygdala. So, lesions in this area of the brain either by drugs may cause decreased dopamine release thereby decreasing motivation.
This cueing procedure, in which the escape platform protrudes above the water surface, provides a control for the animal tested [21]. Here also, the swim latencies were used for the comparison. Mice treated with imipramine and amitriptyline did not show any difference in the swim latencies compared to the control group. The test drugs, therefore, did not negatively affect the motivation to escape and also did not affect the visual integrity of the mice.
The Novel Object Recognition Task (NORT) was originally developed for animal models as a test of declarative memory after it was suggested, that mice would spend more time investigating new objects than the familiar ones [20]. It has since been validated as a test of recognition memory in mice [24,25]. The novel object recognition task has become a widely used model for the investigation into memory alternations. However, it can be configured to measure working, memory, attention, anxiety, and preference for novelty in rodents [26,27]. Yet it has also been used to test the effects of various pharmacological treatments and brain damage [27]. It is important to note that object recognition in animals may be measured by the difference in exploration time of novel and familiar objects. The recognition measure is influenced by the interval between time spent with the novel object and time spent with the sample object as well as the time allowed for mice to explore the sample in a first trial. Thus, a wider range of variables can be sensitive to brain lesions [20]. As mentioned, when animals are exposed to a familiar and a novel object, they approach frequently and spend more time exploring the novel than the familiar one [28]. Following administration of low and high doses of imipramine and amitriptyline, the index of habituation in the short-term memory was significantly lowered in the group treated with low dose (1.8 mg/kg) imipramine and amitriptyline compared to their control. Also, the index of habituation in long-term memory was significantly lower in all test groups compared to the control group.
The index of discrimination in the short term did not differ in the treated group compared to the control group. The index of discrimination was significantly lower in the group treated with low and high doses (1.8 and 3.7 mg/kg) of imipramine and amitriptyline. This result is not in tandem with the Morris Water Maze as the novel object recognition task showed impaired cognitive memory function as observed in this study.
The two antidepressants; imipramine and amitriptyline impaired cognitive memory and enhanced visuospatial learning and memory functions in a dose-dependent manner. Anticholinergic properties of Imipramine and amitriptyline may have been responsible for the impairment of cognitive memory in mice. Cognitive impairment induced by the drugs during treatment might not be a transient effect but may last as long as treatment continues. Patients on these drugs may be at an increased risk of confusion and possibly dementia, especially in high dosages, but more research needs to be done to ascertain this.
- Osim EE. Neurophysiology. 3rd ed. Calabar: University of Calabar Printing Press. 2008; 99.
- Atkinson RC, Shiffrin RM. Human Memory: A proposed system and its control processes. In: Spence KW, Spenc JT, editors. The Psychology of Learning and Motivation 2. New York: Academic Press. 2006; 89-105.
- Eysenck A. Fundamentals of cognition. New York: Psychology Press. 2007; 16-19.
- Baddely A. Working memory, thought and action. Oxford, UK: Oxford University Press. 2007; 30-35.
- Mfem CC, Seriki SA. Impact of amitriptyline on learning and memory. Insights Depress Anxiety. 2021; 5: 009-015. DOI: 10.29328/journal.ida.1001025
- Spear NE, Ricco DC. Memory: Phenomena and principles. Allyn and Bacconprinters. 1994; 156-17.
- Zola M, Suire LR. Neuroanatomy of memory. Ann Neurosci. 2003; 16:547-563.
- Hirschfeld RM. The epidemiology of depression and the evolution of treatment. Journal of Clinical Psychiatry. 2012; 73(Suppl 1): 5–9.
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990-2020: Global Burden of Disease Study. Lancet. 1997 May 24;349(9064):1498-504. doi: 10.1016/S0140-6736(96)07492-2. PMID: 9167458.
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008 Jan 3;358(1):55-68. doi: 10.1056/NEJMra073096. PMID: 18172175.
- Hirschfeld RM. The epidemiology of depression and the evolution of treatment. J Clin Psychiatry. 2012;73 Suppl 1:5-9. doi: 10.4088/JCP.11096su1c.01. PMID: 22951236.Widlocher, D. J. (1983). Psychomotor retardation: clinical, theoretical, and sychometric aspects. Psychiatric Clinical North American, 6(1): 27–40
- Lane MD, O’ Hanlon JF. Cognitive and psychomotor effects of antidepressants with emphasis on selective serotonin reuptake inhibitors and the depressed elderly patient. Ger. J. Psych. 1999; 2:1–42.
- Montenegro M, Veiga H, Deslandes A, Cagy M, McDowell K, Pompeu F, Piedade R, Ribeiro P. Neuromodulatory effects of caffeine and bromazepam on visual event-related potential (P300): a comparative study. Arq Neuropsiquiatr. 2005 Jun;63(2B):410-5. doi: 10.1590/s0004-282x2005000300009. Epub 2005 Jul 25. PMID: 16059590.
- Cunha M, Portela C, Bastos VH, Machado D, Machado S, Velasques B, Budde H, Cagy M, Basile L, Piedade R, Ribeiro P. Responsiveness of sensorimotor cortex during pharmacological intervention with bromazepam. Neurosci Lett. 2008 Dec 19;448(1):33-6. doi: 10.1016/j.neulet.2008.10.024. Epub 2008 Oct 14. PMID: 18938214.
- Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996 Jan;29(1):23-6. doi: 10.1055/s-2007-979537. PMID: 8852530.
- Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry. 2002 Apr;159(4):663-5. doi: 10.1176/appi.ajp.159.4.663. PMID: 11925309.
- Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased brain GABA concentrations following acute administration of a selective serotonin reuptake inhibitor. Am J Psychiatry. 2004 Feb;161(2):368-70. doi: 10.1176/appi.ajp.161.2.368. PMID: 14754790.
- Riga MS, Sánchez C, Celada P, Artigas F. Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: Focus on glutamatergic and GABAergic neurotransmission. Neuropharmacology. 2016 Sep;108:73-81. doi: 10.1016/j.neuropharm.2016.04.023. Epub 2016 Apr 20. PMID: 27106166.
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988 Nov 1;31(1):47-59. doi: 10.1016/0166-4328(88)90157-x. PMID: 3228475.
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984 May;11(1):47-60. doi: 10.1016/0165-0270(84)90007-4. PMID: 6471907.
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994 May;61(3):260-70. doi: 10.1016/s0163-1047(05)80009-3. PMID: 8067981.
- Dalm S, Grootendorst J, de Kloet ER, Oitzl MS. Quantification of swim patterns in the Morris water maze. Behav Res Methods Instrum Comput. 2000 Feb;32(1):134-9. doi: 10.3758/bf03200795. PMID: 10758671.
- Brown RE, Corey SC, Moore AK. Difference in Measures of exploration and fear in MHC congenic C%&BL/6J and B6.H-2k Mice-behaviour Genetics. 1999; 26: 263-271.
- Podhorna J, Brown RE. Strain differences in activity and emotionality do not account for differences in learning and memory performance between C57BL/6 and DBA/2 mice. Genes Brain Behav. 2002 May;1(2):96-110. doi: 10.1034/j.1601-183x.2002.10205.x. PMID: 12884980.
- Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A. Performance of different mouse strains in an object recognition task. Behav Brain Res. 2003 Dec 17;147(1-2):49-54. doi: 10.1016/s0166-4328(03)00117-7. PMID: 14659569.
- Goulart BK, de Lima MN, de Farias CB, Reolon GK, Almeida VR, Quevedo J, Kapczinski F, Schröder N, Roesler R. Ketamine impairs recognition memory consolidation and prevents learning-induced increase in hippocampal brain-derived neurotrophic factor levels. Neuroscience. 2010 Jun 2;167(4):969-73. doi: 10.1016/j.neuroscience.2010.03.032. Epub 2010 Mar 22. PMID: 20338225.
- Selvaraj V, Veeravalli S, Ramaswamy S, Balon R, Yeragani VK. Depression, suicidality and antidepressants: A coincidence? Indian J Psychiatry. 2010 Jan;52(1):17-20. doi: 10.4103/0019-5545.58890. PMID: 20174513; PMCID: PMC2824975.
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988 Nov 1;31(1):47-59. doi: 10.1016/0166-4328(88)90157-x. PMID: 3228475.