More Information
Submitted: May 07, 2024 | Approved: May 14, 2024 | Published: May 15, 2024
How to cite this article: Ajao FO, Kalejaiye NO, Iyedupe MO, Abiodun S, Gbadero J, et al. Cardioprotective Potentials of Anacardium occidentale Nuts Methanolic Extract in Diabetes-Induced Cardiac Dysfunction in Rats. Arch Pharm Pharma Sci. 2024; 8: 056-066.
DOI: 10.29328/journal.apps.1001057
Copyright License: © 2024 Ajao FO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction inany medium, provided the original work is properly cited.
Keywords: Anacardium occidentale nuts; Diabetes mellitus; Oxidative stress & inflammation; Cardiac enzymes & lipid profile; Cardiac apoptosis
Cardioprotective Potentials of Anacardium occidentale Nuts Methanolic Extract in Diabetes-Induced Cardiac Dysfunction in Rats
Folasade Omobolanle Ajao1*, Noheem Olaoluwa Kalejaiye1, Marcus Olaoye Iyedupe1, Sunday Abiodun1, Joy Gbadero1, Pelumi Ogundele1, Zainab Adeagbo1, Oluwatosin Ojolo1, Enitan Shonde1 and Funmilayo Elizabeth Olaleye2
1Physiology Department, Faculty of Basic Medical Science, College of Health Science, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Oyo State, Nigeria
2Department of Human Nutrition and Dietetics, College of Medicine, University College Hospital, Ibadan, Oyo State, Nigeria
*Address for Correspondence: Folasade Omobolanle Ajao, Physiology Department, Faculty of Basic Medical Science, College of Health Science, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Oyo State, Nigeria, Email: [email protected]
Background: The unwanted adverse toxicity displayed by synthetic antidiabetic medicine leads to the search for effective natural medicine to combat diabetes complications. This study investigated the cardioprotective of Anacardium occidentale nuts methanolic in high-fat diet (HFD)/streptozotocin (STZ)-induced diabetic rats.
Materials and methods: Forty male adult Wistar were used and fed with HFD for 6 weeks before diabetes induction. The rats were grouped into 5 groups, 8 rats/group. Group I: normal control; Group II: diabetic control; Group III & IV: diabetic rats + 100 mg/kgb.wt & 200 mg/kgb.wt Anacardium occidentale nuts methanolic extract; Group V: diabetic rats + 200 mg/kgb.wt metformin. The rats were sacrificed on the experiment’s last day, blood samples were collected and the hearts were isolated for biochemical parameters estimation.
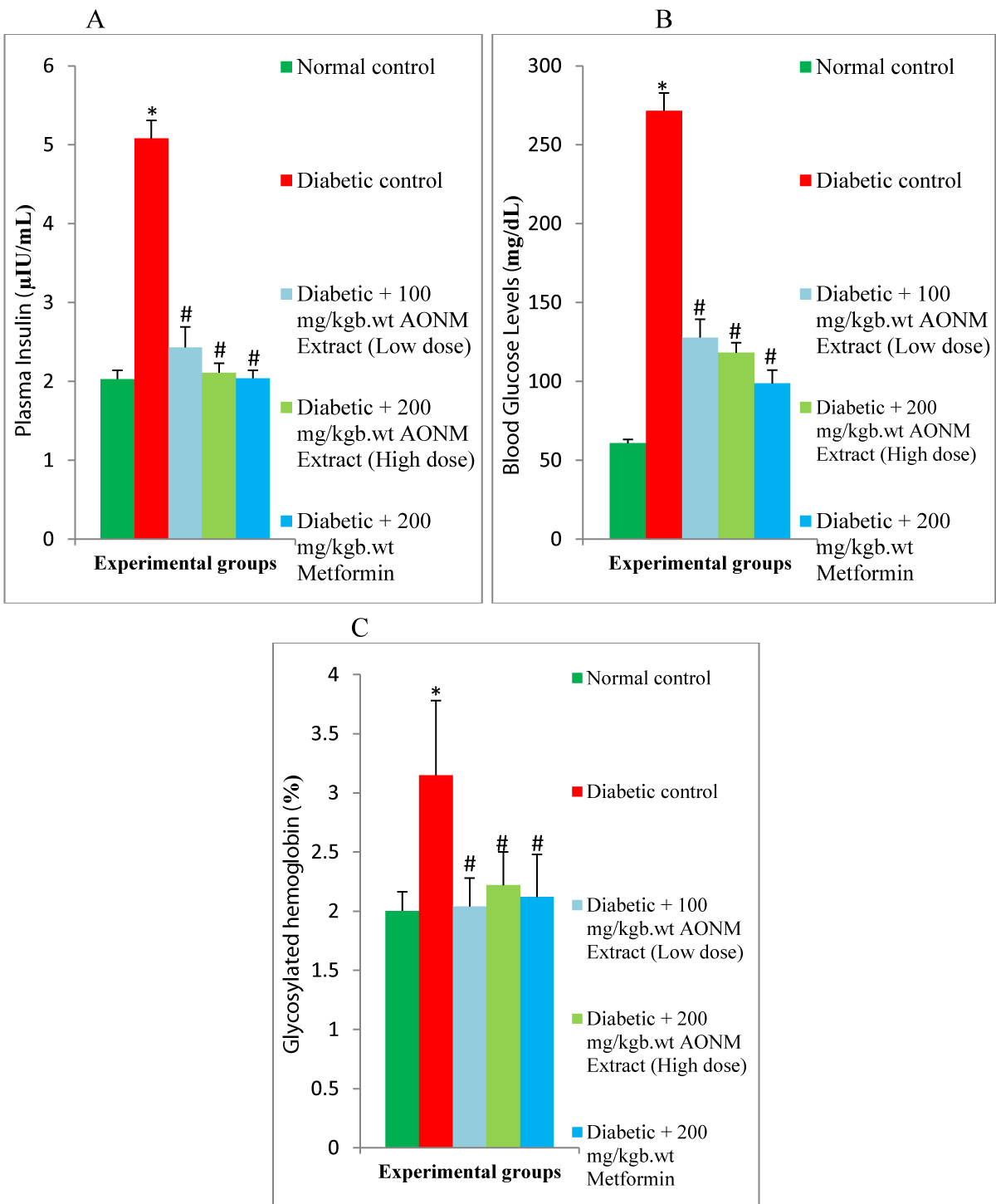
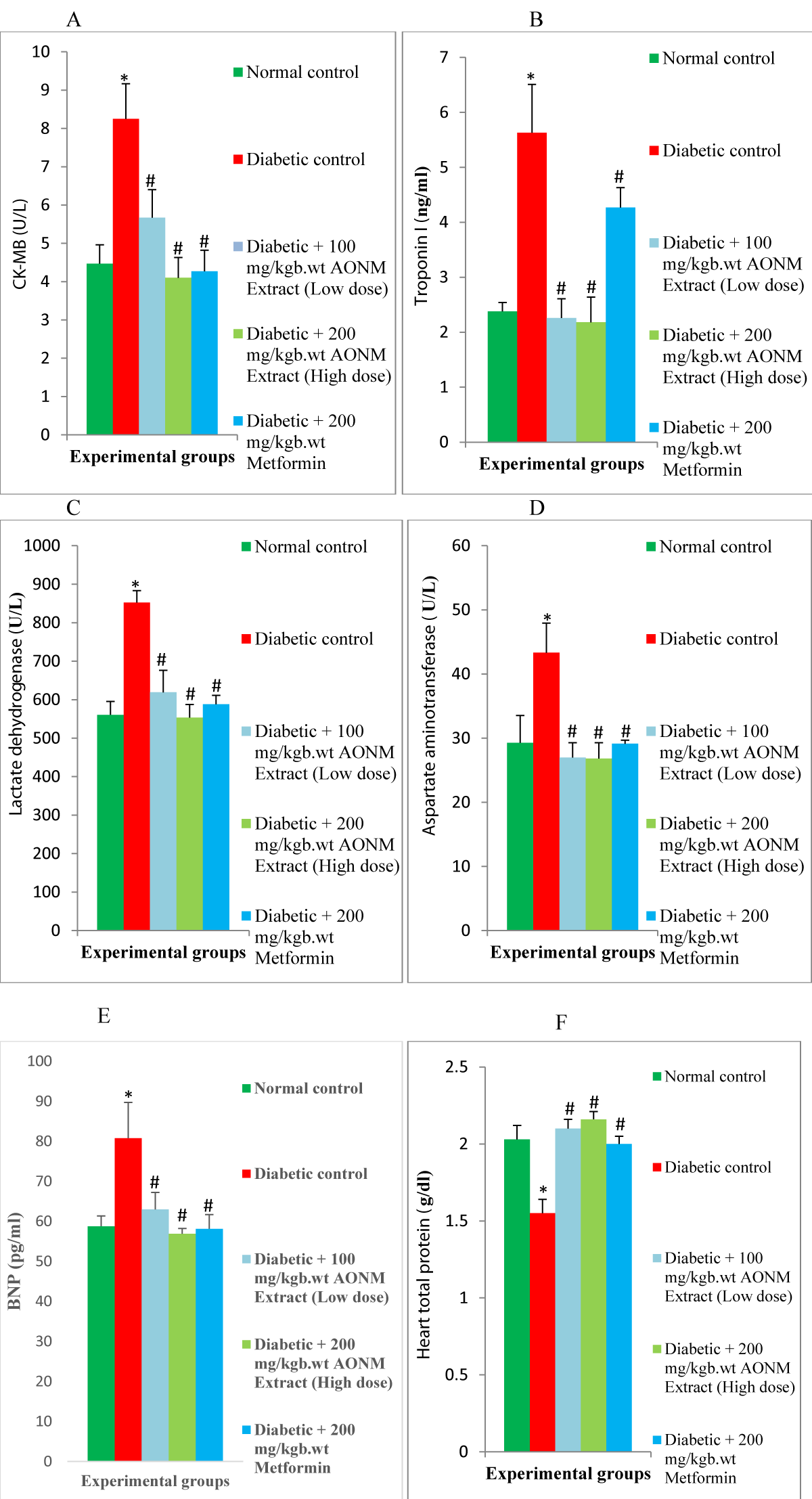
Results: Food intake, water intake, plasmas insulin, Fasting Blood Glucose (FBG), glycosylated hemoglobin (HbA1c), cardiac enzymes, lipid profile, inflammatory cytokines, malondialdehyde, fibrotic marker, caspase-3 in cardiac of diabetic rats were elevated (p < 0.05) significantly. Body weight, cardiac antioxidant, and anti-apoptotic marker levels diminished (p < 0.05) significantly in diabetic rats. 100 mg/kgb.wt & 200 mg/kgb.wt of Anacardium occidentale nuts methanolic extract administration significantly suppressed the plasma insulin, FBG, HbA1c, cardiac lipid profile, cardiac enzymes biomarker, cardiac inflammatory cytokines, cardiac malondialdehyde, cardiac fibrotic marker, cardiac caspase-3, food intake & water intake and increased the body weight, cardiac antioxidant & cardiac anti-apoptotic marker in the diabetic rats.
Conclusion: Anacardium occidentale nuts attenuate cardiac injury in diabetes. It could be a natural medicine to manage diabetes-cardiovascular complications.
The prevalence of diabetes mellitus escalates significantly [1]. According to the International Diabetes Federation, it is projected that diabetes will affect approximately 783.2 million individuals by 2045 [2].
Diabetes mellitus is described as a chronic metabolic disorder due to impairments in pancreatic β-cells insulin secretion, insulin action, or both which disrupt the metabolism of carbohydrates, lipids, and proteins, leading to chronic hyperglycemia [3-5]. Noticeably, untreated sustained hyperglycemia is implicated in the pathogenesis of diabetes-related macro-vascular and micro-vascular complications, including nephropathy, neuropathy, retinopathy, and cardiovascular diseases [6].
Cardiovascular complications are recognized as the foremost cause of mortality and morbidity in type 1 and type 2 diabetic patients [7]. Cardiovascular complications cause 80% of mortality in diabetes conditions [8].
Diabetes treatment strategies in recent decades have advanced. Nevertheless, anti-diabetic medications have severe side effects [9]. The World Health Organization (WHO) has shifted attention to the use of medicinal plants to manage diabetes mellitus [10,11]. Medicinal plants possess notable anti-diabetic compounds including flavonoids, alkaloids, phenolics, and tannins with few or no adverse effects [12].
Anacardium occidentale Linn (A. occidentale) globally known as the cashew tree is a member of the Anacardiaceae family and is grown widely in tropical countries. A. occidentale has been used as a folk remedy for treating a range of diseases including diabetes mellitus [13]. The species of this plant possess abundant phenolic compounds and flavonoids in their leaves, bark, fruits, and nuts. These compounds exhibit potent anti-inflammatory and antioxidant properties, providing cellular protection [14]. Anti-inflammatory, anti-oxidative, and analgesic activities of the nuts have been previously reported [15]. Also, in mild hyperhomocysteinemia rats, oral administration of A. occidentale nuts was reported to counteract biochemical changes, oxidative stress, pro-inflammatory cytokine release, histological tissue injuries, fibrosis, and apoptosis in the kidney, colon, and liver [16]. However, research on A. occidentale nuts to protect and
manage cardiac complications in diabetes has never been elucidated. This recent study scientifically investigated the potential of A. occidentale nuts methanolic extract to attenuate cardiac injury in high-fat diet/streptozotocin-induced diabetic rats.
Chemicals and Drugs: Streptozotocin, methanol, glucose, phosphate buffer, ketamine, and xylazine.
Experimental animals
Forty adult Wistar rats weighing (250 g – 300 g) were purchased from the Animal Research House of the Physiology Department, Ladoke Akintola University of Technology (LAUTECH), Ogbomoso, Oyo State, Nigeria. The animals were kept in a cleaned propylene cage and fed with standard pelletized feed with water ad libitum for 7 days to acclimatize under the pathogen-free environmental conditions of temperature (25 ± 2 °C), relative humidity (45% ± 5%) and 12:12 hour’s light/dark cycles. All experimental procedures were conducted according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals protocol and approved by the Faculty of Basic Medical Science Ethics Research Committee Ladoke Akintola University of Technology (Ethical Approval Number: ERCFBMSLAUTECH:021/01/2024).
A. occidentale nuts collection and extraction
A. occidentale plant was identified at LAUTECH Agriculture Research Farm, authenticated, and assigned a voucher number LH0533 by Dr. A. T. J. Ogunkunle of the Biology Department, LAUTECH. The freshly plunged A. occidentale nuts were washed thoroughly with distilled water, air-dried, and removed from the outer coat before extraction. The nuts were grinded to powder form, kept in an air-tight container, and 500 g of the nut’s powdered form was extracted with 95% methanol in a Soxhlet apparatus to form a semi-solid. The semi-solid was then evaporated in a rotary evaporated pressure and the solid formed was collected and stored at -4 °C until needed.
Diabetes induction
The animals were fed with a High-Fat Diet (HFD) for 6 weeks before diabetes induction. Then, subjected to overnight fasting (12 hours) before diabetes. The animals were injected intraperitoneally with a repeated single dose of freshly prepared streptozotocin (35 mg/kgb.wt) to induce diabetes and given a 2% glucose solution to prevent drug-induced hypoglycemic death. Diabetes induction was authenticated after 72 hours of streptozotocin injection via the animals’ tail prick venous blood using a glucometer (Accu-check) and test stripes. Animals with fasting blood glucose ≥ 200 mg/dL were confirmed diabetic and picked for the experimental study.
Experimental animal grouping
Forty rats were grouped into five groups, 8rats/group. Group I served as control rats, and Group II-V were HFD/STZ-induced diabetic rats treated with different doses of A. occidentale nuts methanolic extract as follows:
Group I: Normal control
Group II: Diabetic control
Group III: Diabetic + 100 mg/kgb.wt AONM Extract (Low dose)
Group IV: Diabetic + 200 mg/kgb.wt AONM Extract (high dose)
Group V: Diabetic + 200 mg/kgb.wt Metformin (reference drug)
A. occidentale nuts methanolic extract was administered for consecutive 6 weeks. Food intake, water intake, and body weight were recorded daily with weighing balance. Weekly fasting blood glucose levels were measured using the glucose-oxidase/peroxidase (GOD-POD) method via the pricked tail vein blood with a digital Accu-Chek glucometer and test strips and recorded throughout the treatment period of the experiment.
Blood Pressure and Electrocardiographic (ECG) parameters recording
The mean Systolic Blood Pressure (SBP) and Diastolic Blood Pressure (DSP) were measured using a non-invasive tail-cuffed method.
ECG was recorded using a three-lead non-invasive with electrodes positioned in lead II and sampled at 1 kHz. RR interval, PR interval, P-wave, QRS complex, QT interval, and heart rate were measured. QT was corrected from the QR interval using the Bazett formula [17]:
where f = 150 ms
Biochemical assay
At the end of the treatment period, the rats were fasted overnight after the last A. occidentale nuts methanolic extract low and high doses (100 mg/kgb.wt & 200 mg/kgb.wt) were administered. The animals were anesthetized with ketamine (40 mg/kgb.wt) and xylazine (20 mg/kgb.wt) and sacrificed by cervical dislocation. Fasting blood samples were collected from the apex beat of the rats’ hearts and the hearts were isolated immediately after blood collection, rinsed in normal saline, and homogenized with freshly prepared cold phosphate-buffered. The blood samples were centrifuged at 3,500 rpm for 15 minutes at 4 °C and the heart tissues homogenates were centrifuged at 10000 rpm for 10 minutes at 4 °C. After centrifugation, the clear supernatant plasma obtained was used for biochemical parameters determination.
Glycated hemoglobin (HbA1c) was estimated using a rat hemoglobin HbA1c assay kit following the manufacturer’s instructions.
Enzyme-Linked Immunosorbent Assay (ELISA) was utilized to measure the levels of insulin, total protein, Creatine Kinase-Myocardial Band (CK-MB), cardiac troponin I, Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Tumor Necrosis Factor-alpha (TNF-α), Brain Natriuretic Peptide (BNP), Transforming Growth Factor-β1 (TGF-β1), B-cell lymphoma-2 (Bcl-2) and caspase-3 in rats. Each assay employed a specific ELISA kit designed for rats, following the manufacturer’s instructions.
Lactate Dehydrogenase (LDH) and aspartate aminotransferase (AST) were determined using a spectrophotometer and assay method with an available commercial kit.
Cardiac lipid profile including Total Cholesterol (TC), Triglycerides (TG), and High-Density Lipoprotein cholesterol (HDL-C), was determined using enzymatic colorimetric methods with commercially available assay kits, following the manufacturer’s protocol. Low-Density Lipoprotein Cholesterol (LDL-C) was calculated using the Friedewald equation: LDL-C = TC - (HDL-C + TG/5) [18]. Cardiovascular Risk Indices (CRI) were calculated as the ratio of TG to HDL-C.
The Atherogenic Coefficient (AC) and Castelli’s Risk Index-1 (CRI-1) were computed using the following formulas:
Atherogenic Coefficient (AC) = (TC - HDL-C)/HDL-C.
Castelli’s Risk Index-1 (CRI-1) = TC/HDL-C.
To determine the cardiac oxidative stress Malondialdehyde (MDA) and antioxidants’ Catalase (CAT) and Superoxide Dismutase (SOD) activities, ELISA assay kits were employed as per the manufacturer’s guidelines.
Statistical analysis
Data were presented as the standard error of means (Mean ± SEM) and were analyzed with a statistical package for social science (SPSS version 21.0 software) using one-way analysis of variance (ANOVA) followed by Tukey’s posthoc test to determine the statistically significant difference between groups. Data at p < 0.05 was considered statistically significant.
Effect of A. occidentale nuts methanolic extract on body weight and relative heart weight in HFD/STZ-induced diabetic rats
Diabetic-induced rats displayed a significant (p < 0.05) reduction in body and relative organ weights compared with normal control rats. The administration of 100 mg/kgb.wt (low dose) and 200 mg/kgb.wt (high dose) A. occidentale nuts methanolic extract improved the body and relative organ weights compared with diabetic control rats (Table 1).