More Information
Submitted: May 22, 2024 | Approved: May 29, 2024 | Published: May 30, 2024
How to cite this article: Yun MY, Baek WJ, Choi HJ. Tussilago farfara Extracts Decrease Lung Injury in Fine Dust-Induced Mice by Inhibiting of Inflammatory Cytokine Levels, Neutrophil Accumulation, and Endothelial Dysfunction. Arch Pharm Pharma Sci. 2024; 8: 067-074.
DOI: 10.29328/journal.apps.1001058
Copyright License: © 2024 Yun MY, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction inany medium, provided the original work is properly cited.
Keywords: Tussilago farfara extracts; Fine Dust (FD); Neutrophils accumulation; Pathological features; Inflammatory cytokines; Lung tissues
Tussilago farfara Extracts Decrease Lung Injury in Fine Dust-Induced Mice by Inhibiting of Inflammatory Cytokine Levels, Neutrophil Accumulation, and Endothelial Dysfunction
Mi-Young Yun1, Won-Jin Baek2 and Hwa-Jung Choi2*
1Department of Beauty Science, Kwangju Women’s University, 40 Yeodae-gil, Gwangsan-gu, Gwangju 62396, South Korea
2Department of Beauty Art, 142 Bansong Beltway (Bansong-dong), Busan 48015, Youngsan University, South Korea
*Address for Correspondence: Hwa-Jung Choi, Department of Beauty Art, 142 Bansong Beltway (Bansong-dong), Busan 48015, Youngsan University, South Korea, E-mail: [email protected]
Fine Dust (FD) in the respiratory air generates a variety of human disease issues throughout the earth. This study aimed to investigate whether (1) Tussilago farfara extracts (TF) decrease neutrophils accumulation, typical pathological features, and goblet cell hyperplasia in mice following exposure to fine dust (FD); (2) inflammatory cytokines result from FD exposure; and (3) asymmetric dimethyl-arginine (ADMA) and symmetric dimethyl-arginine (SDMA) levels in the mice following exposure to FD. Seven-week-old male Balb/c mice (n = 5/group) were instilled two times by intra-nasal-trachea (INT) injection for 3 days and 6 days to the mice four groups; normal, control, FD + dexamethasone (Dexa, positive control), and FD + TF groups. TF suspended in 0.5% carboxymethyl cellulose (CMC) was administered orally to the mice daily for 10 days (100 mg/kg). Neutrophil accumulation, typical pathological features, goblet cell hyperplasia, ADMA, and SDMA levels were assessed on day 10 in FD-induced mice. Results indicated FD significantly reduced neutrophil accumulation in BALF, typical pathological features containing goblet cell hyperplasia in lung tissues, and inflammatory cytokines [interleukin (IL)-17 and tumor necrosis factor-α (TNF-α), macrophage inflammatory protein-2 (MIP-2) and C-X-C motif chemokine 1 (CXCL-1)]. Furthermore, TF significantly decreased levels of elevated ADMA and SDMA by FD exposure. Collectively, TF decreased the counts of neutrophils in BALF, histological changes in lung tissues due to downstream secretion of inflammatory cytokines, and levels of ADMA and SDMA. Therefore, TF may be a potential therapeutics for treating FD-associated diseases.
Particulate matter (PM) known as particles smaller than 10.0 μm composed of particles such as exhaust gases, abrasion, and brake [1]. Fine Dust (FD) consists of a mixture of particles of solid and liquid containing various sizes and chemical compositions [2]. Many studies showed that FD is associated with an increase in cardiovascular mortality and morbidity [3]. The short or long exposure to FD in the air promotes the risk of Cardiovascular Disease (CVD), systemic oxidative stress, nervous system imbalance, and inflammation [4].
The FD could easily penetrate and affect the respiratory tract, and continued exposure could result in dramatic issues including several symptoms in the respiratory tract, and inflammatory responses [5]. A study reported the promotion of inflammatory responses such as cytokine release like TNF-α and expression of proteins like COX-2 by FD stimulation in RAW 264.7 macrophage [6]. Excessive inflammations and oxidative stress are reported as the important underlying events in respiratory injury [7].
Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA) have been recognized as toxic non-proteinogenic amino acids in various human diseases [8]. A variety of important biological functions are regulated by ADMA and SDMA [9]. Emerging experimental evidence reports that ADMA and SDMA play a key role in endothelial dysfunction, apoptosis, and impaired immunological function [10-12].
Human bronchial epithelia are exposed to toxic factors such as FD, which can chronic pulmonary infections and respiratory diseases [13]. Many researches focused on the potent activities of natural phytochemicals. Previous studies have reported the protective effects of plant extracts and phenolic compounds against oxidative stress and inflammation induced by FD [14]. (–)-epigallocatechin-3-gallate (EGCG) also reduces skin inflammation and asthma in rats caused by FDP stimulation [15]. We hypothesized that Tussilago farfara could exert potent protective effects against FD.
Tussilago farfara is an important medicinal plant, flowers and leaves are used as medicine materials to alleviate cough and reduce phlegm [16]. Moreover, as a folk medicine, coltsfoot of T. farfara has been reported for its pharmacological activities, including anti-inflammatory, anti-oxidative, anti-microbial, anti-diabetic, neuro-protection, and anti-cancer [17]. Various compounds have been identified from T. farfara, which are sesquiterpenoids, triterpenoids, flavonoids, phenolic acids, chromones, and pyrrolizidine alkaloids [18-20]. Pyrrolizidine alkaloids (PAs) are heterocyclic organic compounds synthesized by plants and T. farfara has had uses in traditional medicine, but the discovery of toxic pyrrolizidine alkaloids in the plant has resulted in liver health concerns [21]. Nevertheless, the effect of the aerial part extracts of T. farfara in mice by FD has not been reported. Therefore, FD mixed with coal, fly ash, and diesel exhausted particle (CFD) was made in this study and we examined the modulating effects of the aerial part extracts of T. farfara on lung injury by FD-induced inflammatory response and neutrophil accumulation in INT (intra-nasal-trachea) injected balb/c mice.
Preparation of T. farfara
The aerial part of T. farfara was purchased from the Daejeon oriental herb mall (Daejeon, South Korea). The aerial part of T. farfara was sliced into pieces and the 200 g among them mixed with ethanol 2.0 L for 7 days at room temperature. The filtration was conducted with 0.45 μm filter paper and the filtrate was concentrated by freeze‑dryer (EYELA FDU-2100, Japan) and stored at –20 °C.
Preparation of FD
The FD is made with a mixture of CFD possessing a diameter of 10 micrometers (PM10). The FD solution was made with DMSO at 5 mg/mL for coal, 10 mg/mL for fly ash, and 5 mg/mL for diesel-exhausted particles, respectively. Coal power plants generate electricity by burning pulverized coal, which creates a hazardous byproduct known as fly ash. Fly ash contains aluminous and siliceous components The FD is made with Alum (Aluminium Hydroxide Gel Adjuvant) at a final concentration of 8%.
Animal and treatment
The male Balb/c mice (7 weeks) were obtained from Central Lab Animal Inc (Seoul, South Korea) and maintained in the animal care institution at Daejeon University. The animal experiments were conducted according to protocols approved by the Animal Care Committee of the Institute of Daejeon University, South Korea (No. DJUARB2019-021).
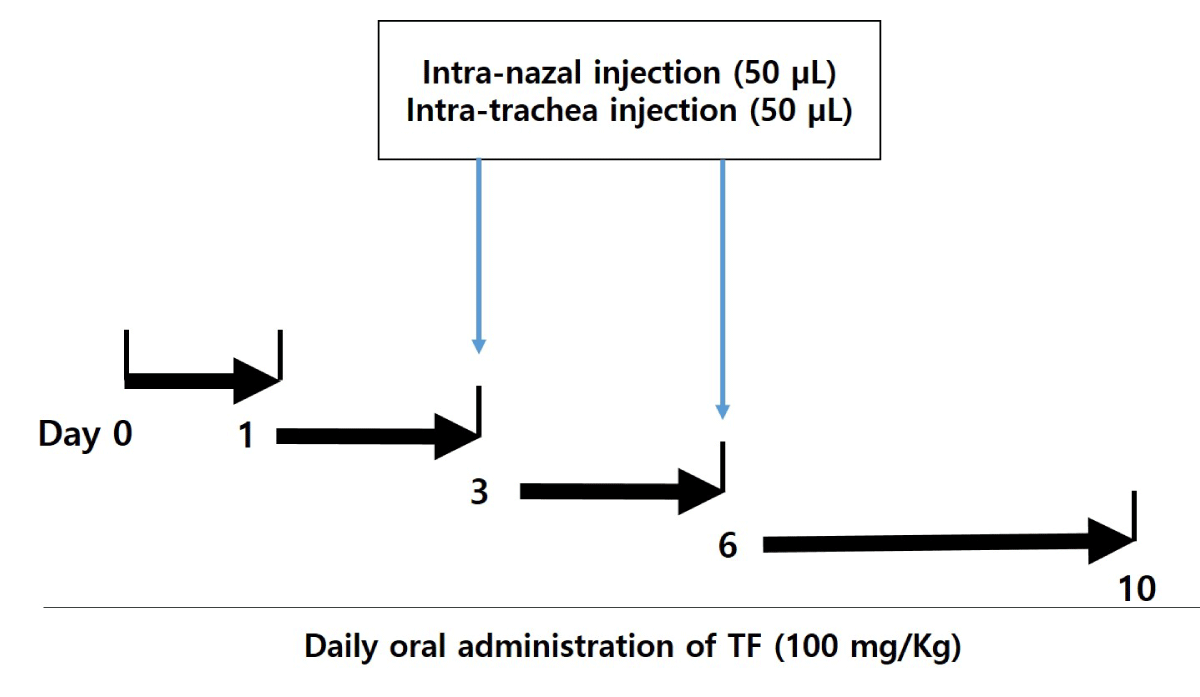
The male mice were divided into 4 groups: normal group (n = 5), control (only treatment with FD) group (n = 5), FD + dexamethasone (Dexa, positive control) group (n = 5), and FD + T. farfara (TF) group (n = 5). After anesthetizing the mice with ketamine, the mice were injected with FD. The respiratory tract of the mice was opened by fixing with a rubber band, the FD was instilled two times by Intra-Nasal-Trachea (INT) injection for 3 days and 6 days the mice (Figure 1). At one time, the FD of 50 μL was instilled with Intra-Nasal (IN) injection, and the other 50 μL instilled with intra-trachea (IT) injection. TF is suspended in 0.5% carboxymethyl cellulose (CMC) and it is administered orally to the mice daily for 10 days (Dose of 100 mg/kg obtained through preliminary experiments).
Figure 1: Experimental scheme of CF-induced mice model. The CF was instilled two times by intra-natal-trachea (INT) injection for 3 and 6 days to Balb/c mice. At one time, the FD of 50 μL was instilled by intra-natal injection, and the other 50 μL was instilled by intra-trachea injection. Tussilago farfara extracts (TF) suspended in 0.5% carboxymethyl cellulose (CMC) treated by oral administration to the mice daily for 10 days (100 mg/kg).
Count of neutrophils in bronchoalveolar lavage fluid (BALF)
The BALF was collected with cannulation of the trachea. Briefly, the neck skin near the trachea of mice was cut with a scalpel, and a catheter was inserted into the trachea. After connecting a syringe of 1 mL to the catheter, 1 mL Phosphate-Buffered Saline (PBS) was injected into the trachea. The BALF was collected for counting neutrophils. BALF cell smears were prepared with cytospin (Thermo Fisher Scientific) and stained with Diff-Quik solution (Dade Diagnostics, Aguada, Puerto Rico) for differential counting on 400 cells to assess neutrophils. The neutrophils were calculated on the cytospin with a Leica microscope under 200 × magnification.
Measurement of inflammatory cytokines in the BALF
The cytokines in the BALF [C-X-C motif chemokine 1 (CXCL-1), interleukin (IL)-17, macrophage inflammatory protein-2 (MIP-2) and tumor necrosis factor-α (TNF-α) were by ELISA with commercial kits (Thermo Fisher Scientific) according to the manufacturer’s protocol. Each cytokine-coated antibody (100 µL) was dispensed into each well and incubated at 4 ℃ for 16 hrs. The plate was washed with washing buffer before the addition of assay diluent (200 µL) and a 1 h incubation at Room Temperature (RT). After diluting the standard solution and diluting the supernatant 20 times, the plate was washed and the standard and supernatant (100 µL) were added to the well and incubated for 2 hrs at RT. The plate was washed, a working detector (100 µL) was added to the well of the plate, and the plate was incubated for 1 h at RT. After another washing, substrate solution (100 µL) was added to wells before incubation in a dark room for 30 min at RT. Finally, stop solution (50 µL) was added to the well and the absorbance was measured at 450 nm on a microplate spectrophotometer.
Asymmetric dimethyl-arginine (ADMA) and symmetric dimethyl-arginine (SDMA)] were quantified by liquid chromatography (LC) with tandem mass spectrometry (LC-MS-MS). Briefly, 25 μL aliquots of plasma were spiked with stable isotope-labeled ADMA, which served as the internal standard. Proteins were precipitated with 100 μL of methanol, filtered through a 0.22 μm hydrophilic membrane (Multiscreen HTS™, Millipore, Molsheim, France), derivatized with butanolic 1 N HCl, and analyzed by LC-tandem MS (Varian 1200 MS, Agilent Technologies, Santa Clara, USA). Quantification was performed by calculation of peak area ratios and calibration with known concentrations of analytes in dialyzed EDTA plasma. The analytical range of the method was validated for 0.05 - 4 μmol/L and the coefficient of variation was ≤ 7.5% both for ADMA and for SDMA.
Histological analysis
After the test for 10 days, the lung tissue of mice was fixed with 10% neutral-buffered formalin, dehydrated, and embedded in paraffin. The lung tissue was cut into 3 μm sections and stained with hematoxylin and eosin (H&E) and periodic acid Schiff (PAS). The stained sections were analyzed under a light microscope (Axio Imager M1; Carl Zeiss, Oberkochen, Germany). The lung inflammation and goblet cell hyperplasia were assessed with a score scale of 0 to 4 [22]. Five scores for each slide were analyzed and the mean score was calculated.
Statistical analysis
The results were recorded as means ± standard deviation (M ± SD). The data was analyzed by ANOVA and Duncan’s multiple range tests. Significance was indicated at p < 0.05, p < 0.01 and p < 0.001. Values are present as the means ± SEMs (n = 5). ### p < 0.001 compared with normal; * p < 0.05, ** p < 0.01 and *** p < 0.001 compared with control. FD is a mixture of coal, fly ash, and diesel-exhausted particles with a diameter of 10 micrometers (PM10 + D).
TF decreased the total count of neutrophils (TCN) in BALF
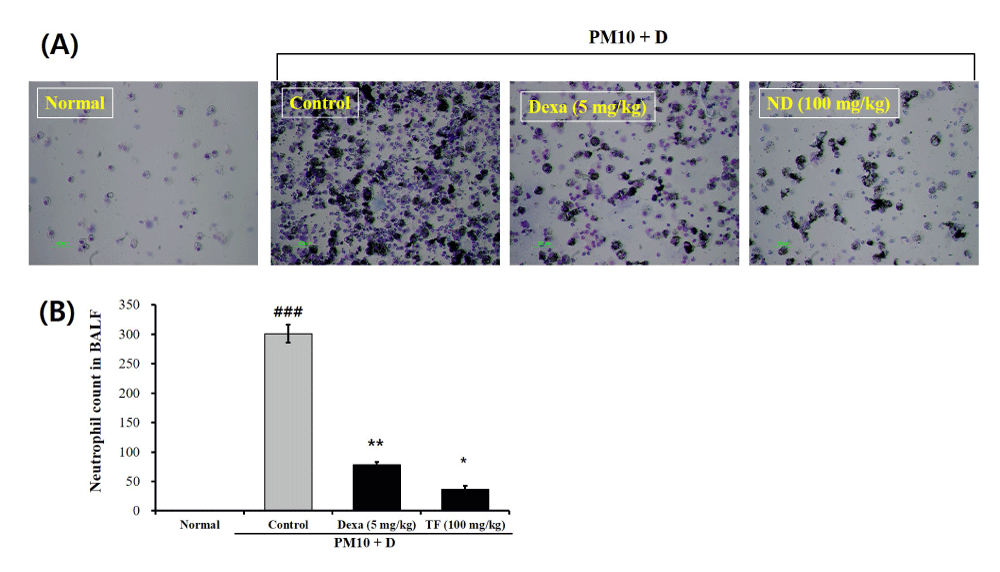
The TCN in the BALF of mice treated with only FD (control group) was significantly higher than the normal group (Figure 2A and 2B). Administration with 5 mg/mL Dexa reduced the TCN of the control group (3.01 × 102) showing the TCN of 7.8 × 101. The TCN in the TF group (3.7 × 101) was significantly lower than the control group (Figure 2A and 2B).
Figure 2: The effects of Tussilago farfara extracts (TF) on neutrophil accumulation in bronchoalveolar lavage fluid (BALF) of FD-induced mice. Diff-Quik™ (International Reagents, Kobe, Japan)-stained smears were used to identify the neutrophil profiles after cytospin preparation. The neutrophil counts were performed by examining 400 cells using a standard light microscope. Normal is normal control. Control is FD-treated control. Dexa is 5 mg/kg dexamethasone. FD is 100 mg/kg FD. Values are present as the means ± SEMs (n = 5). ### p < 0.001 compared with normal; * p < 0.05 and ** p < 0.01 compared with control. FD is a mixture of coal, fly ash, and diesel-exhausted particles with a diameter of 10 micrometers (PM10 + D).
TF decreased lung inflammation and goblet cell hyperplasia
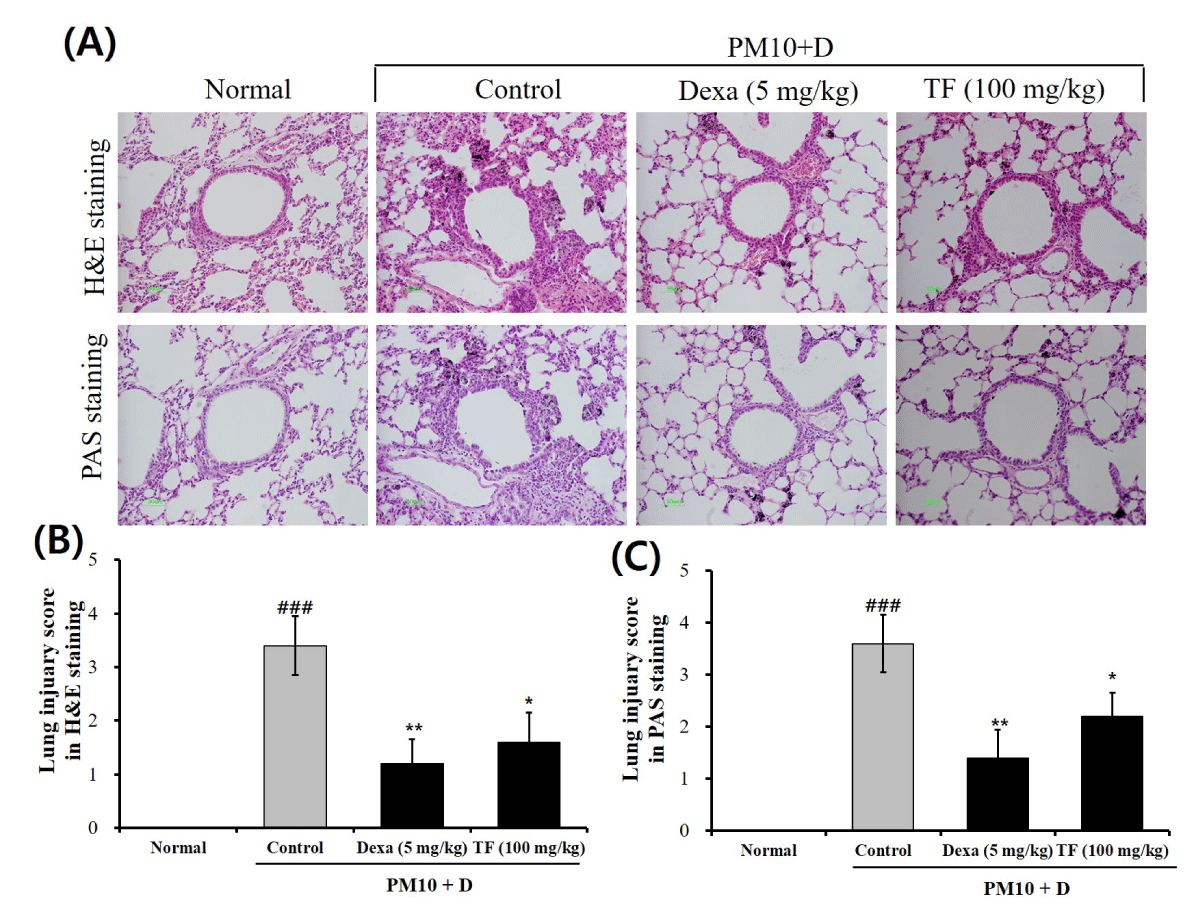
H&E and PAS staining were conducted to observe pathological changes in the lung tissues. In H&E staining, the typical pathological features generated by lung injury showed in control groups as compared to normal groups. The control group showed alveolar ducts, eosinophilic infiltration in pulmonary vessels, and whole lung alveoli (Figure 3A). In contrast, the TF group recovered these symptoms (Figure 3A). PAS staining showed that goblet cell hyperplasia markedly increased in the control group as compared to the normal group (Figure 3A). To evaluate the level of lung injury, the lung injury score in H&E and PAS staining was calculated. As shown in Figures 3B and 3C, both the lung injury scores in H&E and PAS staining were significantly attenuated by the administration of TF. These results indicate that TF improved histopathological change in the FD-induced mice.
Figure 3: Infiltration of inflammatory and goblet cells in lung tissue from FD-challenged mice and TF-treated mice. (A) Representative hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) stained sections of the lung; (B) Lung injury score by histological scoring of inflammatory cell infiltration; (C) PAS score by goblet cells. The lung tissue was stained with H&E and PAS at 10 days after administration of TF. Normal is normal control. Control is FD-treated control. Dexa is 5 mg/kg dexamethasone. ND is 100 mg/kg TF. Values are present as the means ± SEMs (n = 5). ### p < 0.001 compared with normal; * p < 0.05 and ** p < 0.01 compared with control. FD is a mixture of coal, fly ash, and diesel-exhausted particles with a diameter of 10 micrometers (PM10 + D).
TF decreased inflammatory cytokines expression in BALF
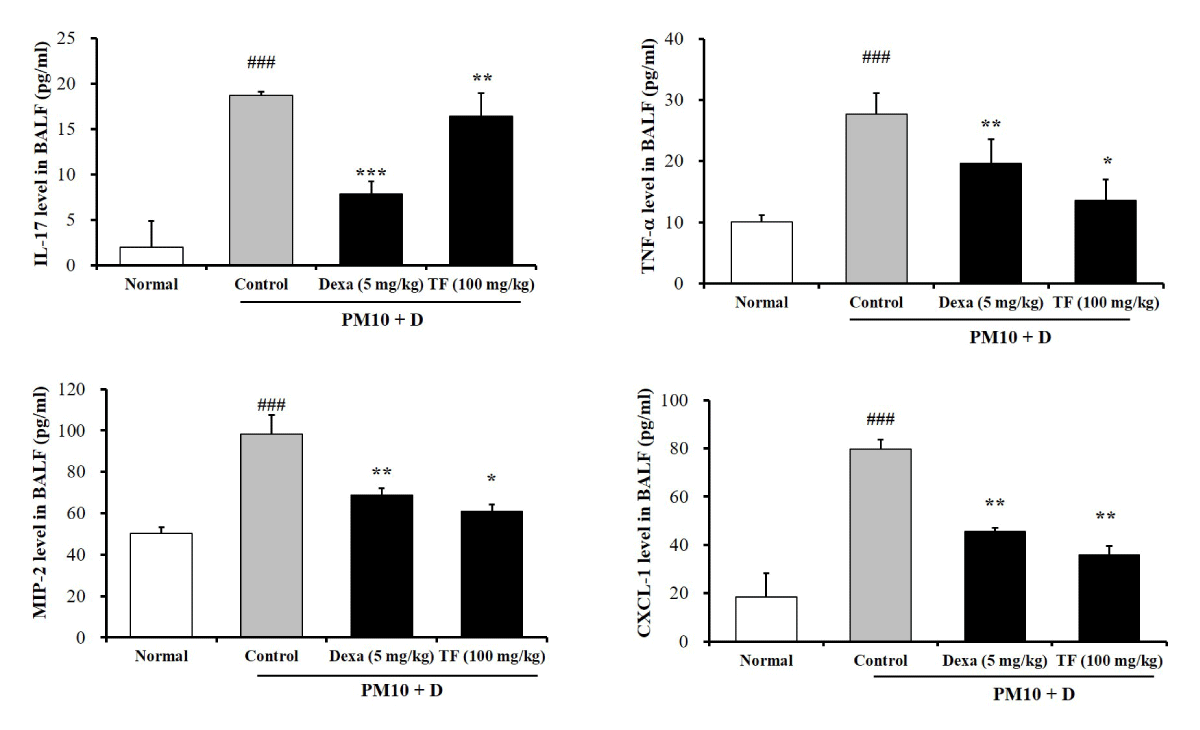
The levels of four inflammatory cytokines (IL-17, TNF-α, MIP-2, and CXCL-1) investigated in the BALF. FD mice increased significantly IL-17, TNF-α MIP-2, and CXCL-1 levels in BALF (Figure 4). TF decreased significantly the levels of IL-17, TNF-α MIP-2, and CXCL-1 (Figure 4).
Figure 4: The effects of Tussilago farfara extracts (TF) on levels of inflammatory cytokines (IL-17, TNF-α, MIP-2, and CXCL-1) in bronchoalveolar lavage fluid (BALF) of FD-induced mice. The inflammatory cytokines levels in the BALF were evaluated by ELISA. Normal is normal control. Control is FD-treated control. Dexa is 5 mg/kg dexamethasone. TF is 100 mg/kg TF. IL-17 is interleukin 17. TNF-α is tumor necrosis factor-α. MIP-2 is macrophage inflammatory protein-2. CXCL-1 is C-X-C motif chemokine 1. Values are present as the means ± SEMs (n = 5). ### p < 0.001 compared with normal; * p < 0.05, ** p < 0.01 and *** p < 0.001 compared with control. FD is a mixture of coal, fly ash, and diesel-exhausted particles with a diameter of 10 micrometers (PM10 + D).
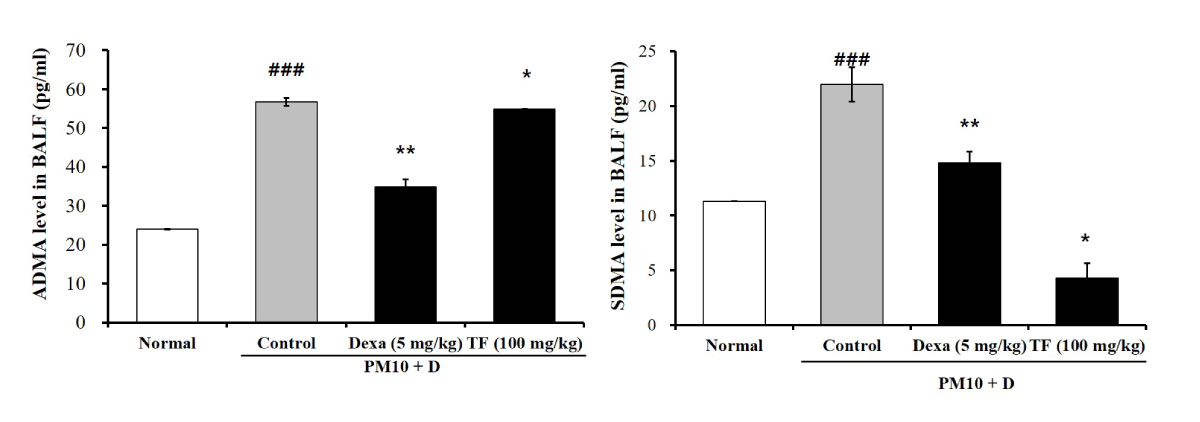
TF decreased ADMA and SDMA in BALF
To investigate the effects of TF on endothelial dysfunction in FD-induced lung injury, ADMA and SDMA levels were checked in BALF. As shown in Figure 5, both ADMA and SDMA levels increased by the FD in the control group. Dexa decreased both ADMA and SDMA levels. As observed in Figure 5, TF restored the increased ADMA and SDMA levels to normal.
Figure 5: The effects of Tussilago farfara extracts (TF) on levels of asymmetric dimethyl-arginine (ADMA) and symmetric dimethyl-arginine (SDMA) in bronchoalveolar lavage fluid (BALF) of FD-induced mice. Mice exposed to FD by intra-nasal trachea injection two times in 3-day intervals for 10 days. One time is 50 μL intra-nasal injection and the other 50 μL is an intra-trachea injection. TF was treated by oral administration to the mice daily for 10 days (100 mg/kg). The ADMA and SDMA levels in BALF were obtained from FD-induced mice with an ELISA kit. Normal is normal control. Control is FD-treated control. Dexa is 5 mg/kg dexamethasone. TF is 100 mg/kg TF. Values are present as the means ± SEMs (n = 5). ### p < 0.001 compared with normal; * p < 0.05, ** p < 0.01 and *** p < 0.001 compared with CTL. Control is a mixture of coal, fly ash, and diesel-exhausted particles with a diameter of 10 micrometers (PM10 + D).
Airway inflammation is an element of chronic eosinophilic inflammation and PM contributes to respiratory disease [23]. Furthermore, PM induced IL-17A in the lungs of exposed mice and enhanced allergic asthma via T-helper cell type 17 (TH17)-induced neutrophils [24]. Despite the increasing concern about FD, many trials need to be prepared in various aspects for risk prevention and reduction of FD-induced diseases. T. farfara polysaccharides reduced the expression of programmed death 1 (PD1; CD279) and its ligand PD-L1 (B7H1, CD274) in peripheral blood and tumor tissue lymphocytes in mice with lung carcinoma [25]. The ethanol extract of flower buds of T. farfara attenuated cigarette smoke-induced lung inflammation by regulating NOD-like receptor 3 (NLRP3) inflammasome, erythroid 2-related factor 2 (Nrf2), and nuclear transcription factor-κB (NF-κB) [26]. In this study, we investigated whether TF could reduce lung injury such as neutrophil accumulation in BALF, typical pathological features, and increased goblet cell hyperplasia. TF decreased markedly neutrophil accumulation, typical pathological features, and goblet cell hyperplasia.
The respirable particles or FD penetrate the lung system and reach the lung alveoli to generate Reactive Oxygen Species (ROS) [27]. Workplace exposures, such as diesel exhaust, and PM increase the expression of inflammatory cytokines [28]. Airway epithelial cells play a key role in the immune response against FD and produce pro-inflammatory cytokines in response to FD [29]. In this study, FD increased these inflammatory cytokines (IL-17, TNF-α, MIP-2, and CXCL-1) in BALF. TF effectively reduced levels of these inflammatory cytokines in BALF.
Numerous studies demonstrated elevated ADMA and SDMA levels in a wide spectrum of human diseases [8]. ADMA and SDMA are endogenous modulators of nitric oxide (NO) synthesis and inhibition of NO synthesis by them may inflammatory reaction, as they interfere with NO synthase upregulated by inflammatory cytokines [30]. Studies show that circulating ADMA levels are elevated in patients with Chronic Kidney Disease (CKD) [31]. As in the case of ADMA, elevated SDMA levels are related to endothelial dysfunction such as hypertension [32]. In this point of view, FD decreased elevated levels of ADMA and SDMA in BALF of mice, both effectively reduced increased levels of ADMA and SDMA.
Chemokines, such as CXCL-1 and MIP2 (CXCL-2), are major neutrophil chemoattractants that are produced in the lung in an airway inflammation model induced by FD exposure; an increase in the pulmonary expression of these C-X-C chemokines exacerbated airway inflammation [33]. Both TRPV1 and TRPA1, members of the TRP channel superfamily, play a key part in lung inflammation and proinflammatory factors, such as leukotriene, IL-1α, and TNF-α, which cause primary airway inflammation [34]. Activation of these transient receptor potential (TRP) channels by exposure to environmental lung toxic irritants, such as FD and cigarette smoke, causes coughing by stimulation of nociceptive C-fibers in the airways of humans and animals, and TRP-channel-induced neurogenic inflammation could lead to the progression and symptoms of airway inflammatory illnesses, such as lung fibrosis and allergic asthma [35].
Exposure to environmental hazards, such as FD and cigarette smoke, stimulates proinflammatory mediators, such as IL-1 and TNF-α, which activate the NF-κB transcription factor or MAPK signaling molecules. This process leads to lung inflammation with neutrophil recruitment to the lung via the pulmonary expression of cytokines and neutrophil chemokines such as CXCL-1 and MIP-2 [36]. Consistent with previous reports, our results show that TF alleviates neutrophilic airway inflammation by preventing NF-κB and ERK/p38/JNK MAPK signaling.
The maximum effective dose of T. farfara for the FD-induced chronic inflammatory disease mice in this study is 100 mg/kg. The practical human dose of T. farfara based on body surface area is about 500 mg/day (60 kg adult, 1 - 2 times/day). However, the appropriate dosage for human administration can differ depending on frequency, gender, and age. A limitation of the current study is that it did not test the effects of T. farfara in both sexes of mice. Recently, studies have reported that in humans, females are more susceptible than males to airway inflammation caused by environmental air pollution owing to sex hormone differences [33]. Thus, the effect of T. farfara on female mice should be tested in a future study.
TF decreased the counts of neutrophils in BALF and histological changes in lung tissues including infiltration of inflammatory cells and goblet cell hyperplasia due to downstream secretion of inflammatory cytokines such as IL-17, TNF-α, MIP-2, and CXCL-1. Furthermore, TF decreased levels of ADMA and SDMA elevated by FD. This study has shown that TF ameliorates neutrophilic airway inflammation and lung injury through the downregulation of NF-κB and MAPK signaling pathways in an FD-induced respiratory disease murine model. These results suggest that TF would be a potent candidate for the management of respiratory disorders.
The authors have no conflicts of interest to declare. No funds, grants, or other support was received. In vitro and vivo laboratory work and study design, M.Y.; manuscript preparation and data analysis, W.J.; data analysis and writing; H.J. All authors have read and agreed to the published version of the manuscript. All authors agree with the submission of the manuscript and declare that they have not submitted it to another journal during the review process.
- Vanicela BD, Nebel M, Stephan M, Riethmüller C, Gresser GT. Quantitative analysis of fine dust particles on moss surfaces under laboratory conditions using the example of Brachythecium rutabulum. Environ Sci Pollut Res Int. 2021 Oct; 28(37):51763-51771. doi: 10.1007/s11356-021-14218-5. Epub 2021 May 15. PMID: 33991303; PMCID: PMC8458176.
- Shiwakoti S, Adhikari D, Lee JP, Kang KW, Lee IS, Kim HJ, Oak MH. Prevention of Fine Dust-Induced Vascular Senescence by Humulus lupulus Extract and Its Major Bioactive Compounds. Antioxidants (Basel). 2020 Dec 7; 9(12):1243. doi: 10.3390/antiox9121243. PMID: 33297587; PMCID: PMC7762380.
- Lee BJ, Kim B, Lee K. Air pollution exposure and cardiovascular disease. Toxicol Res. 2014 Jun;30(2):71-5. doi: 10.5487/TR.2014.30.2.071. PMID: 25071915; PMCID: PMC4112067.
- Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol. 2015 Nov;12(11):627-42. doi: 10.1038/nrcardio.2015.152. Epub 2015 Oct 13. PMID: 26461967.
- Jayawardena TU, Asanka Sanjeewa KK, Shanura Fernando IP, Ryu BM, Kang MC, Jee Y, Lee WW, Jeon YJ. Sargassum horneri (Turner) C. Agardh ethanol extract inhibits the fine dust inflammation response via activating Nrf2/HO-1 signaling in RAW 264.7 cells. BMC Complement Altern Med. 2018 Sep 10;18(1):249. doi: 10.1186/s12906-018-2314-6. PMID: 30200963; PMCID: PMC6131869.
- Shukla A, Timblin C, BeruBe K, Gordon T, McKinney W, Driscoll K, Vacek P, Mossman BT. Inhaled particulate matter causes expression of nuclear factor (NF)-kappaB-related genes and oxidant-dependent NF-kappaB activation in vitro. Am J Respir Cell Mol Biol. 2000 Aug;23(2):182-7. doi: 10.1165/ajrcmb.23.2.4035. PMID: 10919984.
- Bernard K, Hecker L, Luckhardt TR, Cheng G, Thannickal VJ. NADPH oxidases in lung health and disease. Antioxid Redox Signal. 2014 Jun 10;20(17):2838-53. doi: 10.1089/ars.2013.5608. Epub 2014 Jan 3. PMID: 24093231; PMCID: PMC4026303.
- Schepers E, Speer T, Bode-Böger SM, Fliser D, Kielstein JT. Dimethylarginines ADMA and SDMA: the real water-soluble small toxins? Semin Nephrol. 2014 Mar;34(2):97-105. doi: 10.1016/j.semnephrol.2014.02.003. Epub 2014 Feb 17. PMID: 24780466.
- Tain YL, Hsu CT. Toxic dimethylarginines: Asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA). Toxins (Basel) 2017; 9(3):92. doi: 10.3390/toxins9030092.
- Cooke JP. Does ADMA cause endothelial dysfunction? Arterioscler Thromb Vasc Biol. 2000 Sep;20(9):2032-7. doi: 10.1161/01.atv.20.9.2032. PMID: 10978245.
- Park MJ, Oh KS, Nho JH, Kim GY, Kim DI. Asymmetric dimethylarginine (ADMA) treatment induces apoptosis in cultured rat mesangial cells via endoplasmic reticulum stress activation. Cell Biol Int. 2016 Jun;40(6):662-70. doi: 10.1002/cbin.10602. Epub 2016 Apr 6. PMID: 26992443.
- Pekarova M, Kubala L, Martiskova H, Bino L, Twarogova M, Klinke A, Rudolph TK, Kuchtova Z, Kolarova H, Ambrozova G, Kuchta R, Kadlec J, Lojek A. Asymmetric dimethylarginine regulates the lipopolysaccharide-induced nitric oxide production in macrophages by suppressing the activation of NF-kappaB and iNOS expression. Eur J Pharmacol. 2013 Aug 5;713(1-3):68-77. doi: 10.1016/j.ejphar.2013.05.001. Epub 2013 May 9. PMID: 23665490.
- Kim J, Choi H, Choi DH, Park K, Kim HJ, Park M. Application of green tea catechins, polysaccharides, and flavonol prevent fine dust induced bronchial damage by modulating inflammation and airway cilia. Sci Rep. 2021 Jan 26;11(1):2232. doi: 10.1038/s41598-021-81989-9. PMID: 33500561; PMCID: PMC7838266.
- Knickle A, Fernando W, Greenshields AL, Rupasinghe HPV, Hoskin DW. Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem Toxicol. 2018 Aug;118:154-167. doi: 10.1016/j.fct.2018.05.005. Epub 2018 May 6. PMID: 29742465.
- Wang L, Lee W, Cui YR, Ahn G, Jeon YJ. Protective effect of green tea catechin against urban fine dust particle-induced skin aging by regulation of NF-κB, AP-1, and MAPKs signaling pathways. Environ Pollut. 2019 Sep;252(Pt B):1318-1324. doi: 10.1016/j.envpol.2019.06.029. Epub 2019 Jun 14. PMID: 31252129.
- Yizhong D, Lu K, Zhongyu D, Shen Y. The complete chloroplast genome sequence of Tussilago farfara (Asteraceae). Mitochondrial DNA B Resour. 2022 Mar 25;7(3):528-530. doi: 10.1080/23802359.2021.2005494. PMID: 35356793; PMCID: PMC8959504.
- Chen S, Dong L, Quan H, Zhou X, Ma J, Xia W, Zhou H, Fu X. A review of the ethnobotanical value, phytochemistry, pharmacology, toxicity and quality control of Tussilago farfara L. (coltsfoot). J Ethnopharmacol. 2021 Mar 1;267:113478. doi: 10.1016/j.jep.2020.113478. Epub 2020 Oct 16. PMID: 33069788; PMCID: PMC7561605.
- Röder E, Wiedenfeld H, Jost EJ. Tussilagin - ein neues Pyrrolizidinalkaloid aus Tussilago farfara [Tussilagine - a new pyrrolizidine alkaloid from Tussilago farfara]. Planta Med. 1981 Sep;43(1):99-102. German. doi: 10.1055/s-2007-971485. PMID: 17402021.
- Qin K, Liu CH, Qi YX, Li K. Evaluation of antioxidant activity of polysaccharides from Tussilago farfara L. by flow injection analysis. Asian J Chem. 2014; 26(10):3073–3076. doi:10.14233/ajchem.2014.16685.
- Qu H, Yang W, Li J. Structural characterization of a polysaccharide from the flower buds of Tussilago farfara, and its effect on proliferation and apoptosis of A549 human non-small lung cancer cell line. Int J Biol Macromol. 2018 Jul 1;113:849-858. doi: 10.1016/j.ijbiomac.2018.03.005. Epub 2018 Mar 2. PMID: 29505876.
- Schramm S, Köhler N, Rozhon W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules. 2019 Jan 30;24(3):498. doi: 10.3390/molecules24030498. PMID: 30704105; PMCID: PMC6385001.
- Kujur W, Gurram RK, Haleem N, Maurya SK, Agrewala JN. Caerulomycin A inhibits Th2 cell activity: a possible role in the management of asthma. Sci Rep. 2015 Oct 20;5:15396. doi: 10.1038/srep15396. PMID: 26481184; PMCID: PMC4612543.
- Guan WJ, Zheng XY, Chung KF, Zhong NS. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016 Oct 15;388(10054):1939-1951. doi: 10.1016/S0140-6736(16)31597-5. PMID: 27751401.
- Castañeda AR, Bein KJ, Smiley-Jewell S, Pinkerton KE. Fine particulate matter (PM2.5) enhances allergic sensitization in BALB/c mice. J Toxicol Environ Health A. 2017;80(4):197-207. doi: 10.1080/15287394.2016.1222920. Epub 2017 May 11. PMID: 28494199; PMCID: PMC6159927.
- Safonova EA, Lopatina KA, Razina TG, Zueva EP, Gur'ev AM, Belousov MV. Effects of Tussilago farfara L. Polysaccharides on the Expression of PD-1 (CD279) and PD-L1 (CD274) in Peripheral Blood and Tumor Tissue Lymphocytes in Mice with Lewis Lung Carcinoma. Bull Exp Biol Med. 2020 Jul;169(3):378-382. doi: 10.1007/s10517-020-04891-w. Epub 2020 Aug 4. PMID: 32749562.
- Xu LT, Wang T, Fang KL, Zhao Y, Wang XN, Ren DM, Shen T. The ethanol extract of flower buds of Tussilago farfara L. attenuates cigarette smoke-induced lung inflammation through regulating NLRP3 inflammasome, Nrf2, and NF-κB. J Ethnopharmacol. 2022 Jan 30;283:114694. doi: 10.1016/j.jep.2021.114694. Epub 2021 Sep 30. PMID: 34601084.
- Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health. 2013 Aug 27;10(9):3886-907. doi: 10.3390/ijerph10093886. PMID: 23985773; PMCID: PMC3799517.
- Pacheco KA. Epigenetics mediate environment : gene effects on occupational sensitization. Curr Opin Allergy Clin Immunol. 2012 Apr;12(2):111-8. doi: 10.1097/ACI.0b013e328351518f. PMID: 22306555.
- Esmaeil N, Gharagozloo M, Rezaei A, Grunig G. Dust events, pulmonary diseases and immune system. Am J Clin Exp Immunol. 2014 Feb 27;3(1):20-9. PMID: 24660118; PMCID: PMC3960758.
- Böger RH. Live and let die: asymmetric dimethylarginine and septic shock. Crit Care. 2006;10(6):169. doi: 10.1186/cc5076. PMID: 17094795; PMCID: PMC1794448.
- Liu X, Xu X, Shang R, Chen Y. Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide. 2018 Aug 1;78:113-120. doi: 10.1016/j.niox.2018.06.004. Epub 2018 Jun 19. PMID: 29928990; PMCID: PMC6301111.
- Hermenegildo C, Medina P, Peiró M, Segarra G, Vila JM, Ortega J, Lluch S. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in hyperthyroid patients. J Clin Endocrinol Metab. 2002 Dec;87(12):5636-40. doi: 10.1210/jc.2002-020905. PMID: 12466365.
- Bonser LR, Erle DJ. Airway Mucus and Asthma: The Role of MUC5AC and MUC5B. J Clin Med. 2017 Nov 29;6(12):112. doi: 10.3390/jcm6120112. PMID: 29186064; PMCID: PMC5742801.
- Grace MS, Baxter M, Dubuis E, Birrell MA, Belvisi MG. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol. 2014 May;171(10):2593-607. doi: 10.1111/bph.12538. PMID: 24286227; PMCID: PMC4009002.
- Dietrich A, Steinritz D, Gudermann T. Transient receptor potential (TRP) channels as molecular targets in lung toxicology and associated diseases. Cell Calcium. 2017 Nov;67:123-137. doi: 10.1016/j.ceca.2017.04.005. Epub 2017 Apr 26. PMID: 28499580.
- Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 2005 Dec 1;175(11):7530-5. doi: 10.4049/jimmunol.175.11.7530. PMID: 16301661; PMCID: PMC2723739.