More Information
Submitted: February 29, 2024 | Approved: July 09, 2024 | Published: July 10, 2024
How to cite this article: Ayyad RR, Mansour AM, Nejm AM, Hassan YAA, Gabr NH, Ayyad AR. Beta-1 Receptor (β1) in the Heart Specific Indicate to Stereoselectivity. Arch Pharm Pharma Sci. 2024; 8(1): 082 - 088. Available from: https://dx.doi.org/10.29328/journal.apps.1001060
DOI: 10.29328/journal.apps.1001060
Copyright License: © 2024 Ayyad RR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction inany medium, provided the original work is properly cited.
Keywords: β1 Receptor, Beta Blockers, Stereoselectivity, Propranolol, Atenolol, Metoprolol
Beta-1 Receptor (β1) in the Heart Specific Indicate to Stereoselectivity
Rezk Rezk Ayyad1*, Ahmed Mohamed Mansour2, Ahmed Mohamed Nejm3, Yasser Abdel Allem Hassan4, Norhan Hassan Gabr5 and Ahmed Rezk Ayyad6
1Pharmaceutical Medicinal Chemistry Department, Faculty of Pharmacy, ALAZHAR University, Cairo, Egypt
2Pharmacology and Toxicology Department, Faculty of Pharmacy, ALAZHAR University, Cairo, Egypt
3Student at Faculty of Pharmacy, ALAZHAR University, Cairo, Egypt
4Pharmaceutics and Pharmaceutical Technology Department, Faculty of Pharmacy, Delta University for Science and Technology, Gamasa, Addaqahlya, Egypt
5Student at Faculty of Pharmacy, Hours University, New Damietta, Cairo, Egypt
6Student at Faculty of Medicine, Asfendiyrov, Almaty, Kazakh, National Medical University (KazNMU), Kazakhstan
*Address for Correspondence: Rezk Rezk Ayyad, Pharmaceutical Medicinal Chemistry Department, Faculty of Pharmacy, ALAZHAR University, Cairo, Egypt, Email: [email protected]
The β1 receptor is one of the three beta receptors present in the human body, namely β1, β2, and β3. The β1 receptor is predominantly located in the heart, where it plays a crucial role in regulating the heart rate and the force of contraction, thereby increasing the cardiac output and the efficiency of blood pumping throughout the body. This receptor is targeted by a variety of pharmaceutical agents known as beta-blockers, which are commonly used in the treatment of cardiovascular conditions such as hypertension, angina, and arrhythmias.The β1 receptor exhibits stereoselectivity, meaning that different enantiomers (chiral molecules) of beta blockers can have varying levels of effectiveness and side effects. This study focuses on the stereoselectivity of the β1 receptor and the clinical implications of this property. It includes an examination of various β1 blockers, such as propranolol (a non-selective beta blocker), and selective β1 blockers like atenolol, bisoprolol, nebivolol, metoprolol, esmolol, acebutolol, and betaxolol. Each of these drugs has a unique chemical structure, with specific functional groups that contribute to their selective action on the β1 receptor.
Furthermore, the β2 receptor, which is mainly present in the bronchi and bronchioles, is responsible for bronchodilation, and the β3 receptor, found in the bladder, helps reduce urinary urgency. Understanding the distinct locations and functions of these receptors allows for the development of targeted therapies with minimal off-target effects.
This review highlights the importance of stereoselectivity in the development and use of β1 blockers, discussing their chemical structures, pharmacological activities, and therapeutic uses. It also explores the potential for future research and development of more selective and effective β1 receptor agonists and antagonists, which could offer improved therapeutic outcomes for patients with cardiovascular diseases.
This study underscores the significant role of the β1 receptor in cardiovascular health and provides insights into the ongoing advancements in beta-blocker therapy. By delving into the stereoselectivity and specific actions of these drugs, the research aims to enhance the understanding and optimization of β1 receptor-targeted treatments in clinical practice.
β1: Beta-1 Receptor; β2: Beta-2 Receptor; β3: Beta-3 Receptor; CDK: Cyclin-Dependent Kinase; EGFR: Epidermal Growth Factor Receptor; VEGFR-2: Vascular Endothelial Growth Factor Receptor-2; GABA: Gamma-Aminobutyric Acid; ADMET: Absorption, Distribution, Metabolism, Excretion, and Toxicity; COX: Cyclooxygenase; DNA: Deoxyribonucleic Acid; PKI: Protein Kinase Inhibitor; HCl: Hydrochloride; HPLC: High-Performance Liquid Chromatography; NMR: Nuclear Magnetic Resonance; MRI: Magnetic Resonance Imaging; IV: Intravenous; AMPA: Alpha-amino-3-hydroxy-5-methyl-4-isoxazole Propionic Acid; NSAID: Nonsteroidal Anti-Inflammatory Drug; COX-1/2: Cyclooxygenase-1/2; RNA: Ribonucleic Acid; mRNA: Messenger Ribonucleic Acid; PCR: Polymerase Chain Reaction; RT-PCR: Reverse Transcription Polymerase Chain Reaction; ELISA: Enzyme-Linked Immunosorbent Assay; MS: Mass Spectrometry; UV: Ultraviolet; IR: Infrared; FDA: Food and Drug Administration; EMA: European Medicines Agency; WHO: World Health Organization; GCP: Good Clinical Practice; GLP: Good Laboratory Practice; ICH: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; CRISPR: Clustered Regularly Interspaced Short Palindromic Repeats; CAS: Chemical Abstracts Service; SMILES: Simplified Molecular Input Line Entry System; QSAR: Quantitative Structure-Activity Relationship
Beta receptors are a class of adrenergic receptors that are sensitive to the catecholamines epinephrine and norepinephrine. These receptors are divided into three subtypes: β1, β2, and β3. The β1 receptors are primarily located in the heart and kidneys, where they play a crucial role in regulating cardiac function and renin release. The β1 receptor exhibits stereoselectivity, meaning that different enantiomers of beta blockers can have varying levels of effectiveness and side effects. This principle is also seen in the activity of histaminic receptors, where stereoselectivity plays a crucial role in their anti-histaminic activity [1]. Activation of β1 receptors leads to an increase in heart rate, myocardial contractility, and cardiac output [2]. Furthermore, the stereoselectivity of certain drugs is crucial, as isomers can have varying levels of effectiveness and toxicity [3], additionally, recent studies have explored various synthetic compounds targeting these receptors for their potential therapeutic applications. For instance, the synthesis and biological evaluation of certain oxindole–indole conjugates have shown promising results as anticancer CDK inhibitors [2]. β2 receptors are found mainly in the lungs, gastrointestinal tract, liver, uterus, vascular smooth muscle, and skeletal muscle, where they mediate smooth muscle relaxation, resulting in bronchodilation and vasodilation. The β3 receptors are located in adipose tissue and the bladder, and their activation contributes to lipolysis and the relaxation of the detrusor muscle, respectively [4].
Inhibition of carbonic anhydrase isoforms I, II, IV, VII, and XII with carboxylates and sulfonamides incorporating phthalimide/phthalic anhydride scaffolds has been explored to understand the pharmacological interactions better [5].
In the context of developing novel therapeutic agents, research on various compounds has been extensive. For example, the design and synthesis of novel 2-(3-methyl-2-oxoquinoxalin-1(2H)-yl)-N-(4-(substituted)phenyl) acetamide derivatives have shown potential as anticonvulsant agents. This study aims to further understand the role of β1 receptors and their stereoselectivity in cardiovascular health and the development of selective β1 blockers [6].
Recent studies have explored various quinazolinone derivatives due to their significant pharmacological activities. For instance, new immunomodulatory anticancer quinazolinone-based thalidomide analogs have shown promising results in design, synthesis, and biological evaluation [7]. Furthermore, trimethoxyanilides based on 4(3H)-quinazolinone scaffolds have shown promising antitumor properties, highlighting the therapeutic potential of quinazolinone-based compounds in medicinal chemistry[8]. Similarly, the development of new pyrimidine-5-carbonitrile derivatives as EGFR inhibitors has demonstrated significant anticancer and apoptotic activities, further showcasing the potential of these compounds in cancer therapy [9].
The design and synthesis of new therapeutic agents, targeting specific receptors have shown promising results. Recent advances in drugs targeting protein kinases for cancer therapy highlight the ongoing efforts to improve treatment efficacy [10], furthermore agents, such as quinazolin-4(3H)-ones, targeting specific receptors like VEGFR-2, have shown promising anti-proliferative activities, which are crucial for developing targeted therapies [11]. Additionally, the synthesis and antiproliferative activity of 1-(4-(1H-Indol-3-Yl)-6-(4-Methoxyphenyl) Pyrimidin-2-yl) Hydrazine and its pyrazole pyrimidine derivatives have demonstrated significant potential in cancer treatment [12]. The development of quinazoline-based anticancer agents targeting VEGFR-2 has shown promising anti-proliferative activities, contributing to advancements in targeted cancer therapy [13]. Moreover, studies on the design, synthesis, in‐vivo anti‐diabetic activity, in‐vitro α‐glucosidase inhibitory activity, and molecular docking of some quinazolinone derivatives have also shown promising results in the treatment of diabetes [14]. The design and synthesis of analgesics and anticancer agents from new benzimidazole derivatives have also been explored for their potential therapeutic benefits [15]. Furthermore, the synthesis and biological evaluation of novel iodophthalazinedione derivatives has shown promising anticonvulsant activity [16]. Additionally, the synthesis and biological evaluation of 1-benzyl-5-bromo-3-hydrazonoindolin-2-ones as novel anti-cancer agents have demonstrated significant potential in cancer therapy [17]. Similarly, the design and synthesis of some new oxadiazole derivatives have shown potential as anticancer agents, providing further insights into the development of novel anticancer therapies [18]. Furthermore, the design and synthesis of new compounds derived from phenylhydrazine and different aldehydes have shown promising anticancer activities, adding valuable knowledge to the field of cancer drug development [19]. Moreover, the design, synthesis, computer modeling, and analgesic activity of some new disubstituted quinazoline-4 (3H)-ones have demonstrated significant potential as analgesic agents [20]. Additionally, the synthesis and anticonvulsant activity of 6-iodo phthalazinedione derivatives have shown promising results, highlighting their potential in the treatment of neurological disorders [21].
In this review of the presence of β1 receptor exclusively mainly in the heart and eye (mostly in their heart and to an extent in the eye), we note all Beta blockers have chiral carbon and alkyl group with more than one carbon on the amino group, the β1 blockers have phenoxy group which substituted at its para-position [22].
Recent research has focused on the development of new pharmacological agents that can target these receptors with high specificity and efficacy. For instance, the design and synthesis of new benzylidene-quinazolinone hybrids as potential anti-diabetic agents have shown promising results in vitro, demonstrating significant α-glucosidase inhibition and effective docking studies[23].
Additionally, the design and synthesis of new quinazolinone derivatives containing sulfonylurea have shown significant antihyperglycemic activity, providing new avenues for diabetes treatment [44].
Further research has identified novel compounds, including triazole-quinoxaline-based molecules, which have shown potential immunomodulatory and anticancer activities in their design, synthesis, and biological evaluation [25].
All the mentioned properties of β1blockers lead to the stereospecific of these compounds and the stereoselectivity of the β1 receptor.
The adrinomimetic drugs are act on the adrenomimetic receptors which are α-types and β-types, the first adrenomimetics are noradrenaline and adrenaline natural substances of the body, which synthesized from tyrosine amino acids to form noradrenaline which alkylated the amino group to adrenaline. The noradrenaline and adrenaline know us which of them act on beta receptors more than the others.
Adrenaline is characterized by a methyl group on the amino group (which makes adrenaline act on α and β receptors), while noradrenaline acts mainly on α receptors. Hence, when the group of alkyl in the amino group is called the beta directly group.
So the β receptors required compounds have alkyl. g.p. on the amino group of noradrenaline [26].
Chemistry and pharmacological activity
Furthermore, studies on similar molecular structures have shown promising results in the development of potential therapeutic agents. The activity of antibiotics and their effectiveness can heavily depend on their stereochemistry [27]. For instance, the design, synthesis, molecular modeling, and biological evaluation of novel 2, 3-dihydrophthalazine-1, and 4-dione derivatives have demonstrated significant anticonvulsant activity, providing insights into the potential for similar approaches in β1 receptor-targeted drug development [28].
The development of these β1 blockers has significantly contributed to the management of various cardiovascular conditions. Their distinct chemical structures and hydrophobicity, along with transport and target sites of action, are crucial for their activity and effectiveness [29] and stereoselectivity enables them to selectively target the β1 receptor, leading to improved therapeutic outcomes. Further research has identified novel compounds, including triazolophthalazine derivatives, which function as DNA intercalators and topoisomerase II inhibitors, thereby expanding their potential therapeutic applications beyond cardiovascular diseases [30]. Additionally, the discovery and antiproliferative evaluation of new quinoxalines as potential DNA intercalators and topoisomerase II inhibitors have shown promise in enhancing the efficacy and safety of these treatments [31]. The mechanism of action for many drugs depends on their ability to inhibit specific enzymes, which is a critical factor in their pharmacological activity [32]. The configuration of hormonal compounds plays a significant role in their pharmacological action, influencing their activity as agonists, antagonists, and their overall efficacy [13].
The β1 receptor exhibits stereoselectivity meaning that different enantiomers (chiral molecules) of beta blockers can have varying levels of effectiveness and side effects [33]. This principle is also seen in the activity of histaminic receptors, where stereoselectivity plays a crucial role in their anti-histaminic activity [34].
Further research and development in this field may uncover additional β1 receptor agonists with enhanced efficacy and fewer side effects providing even more options for patients with cardiovascular disorders. Studies have shown significant advancements in molecular docking and anticancer evaluations of novel compounds, demonstrating promising therapeutic potentials [35].
In recent studies, novel compounds such as triazolo [3,4-a] phthalazine derivatives targeting the VEGFR-2 enzyme have shown promising in vitro anti-cancer activity and favorable ADMET profiles [36].
The lipid solubility of drugs plays an important role in their pharmacological action and duration of action, affecting their overall efficacy and bioavailability [37].
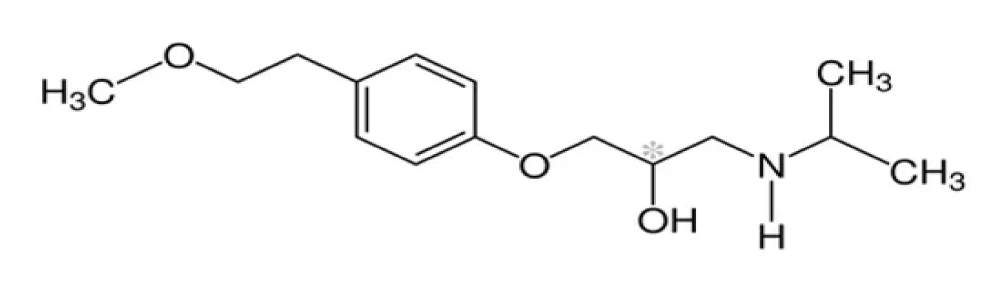
Propranolol
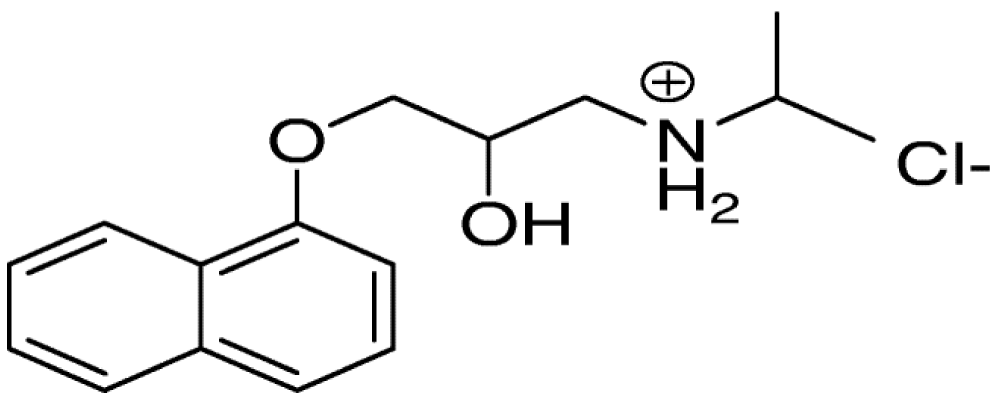
Propranolol is a non-specific beta-blocker (contraindicated in bronchial asthma – asthmatic patients), propranolol is used in the treatment of hypertension, irregular heartbeat, thyrotoxicosis, essential tremors, performance anxiety, capillary heme angioma, migraine headache, angina, heart attacks. The drug used intravenous injection and orally which is short-acting or long-acting. Propranolol is a naphthyloxy derivative and has an isopropyl group on the amino group they share in the non-specificity of the drug. Lastly, propranolol is (a prototype of β- blocker – lead compound). Propranolol is a naphthyloxy derivative and has an isopropyl group on the amino group, contributing to its non-specificity (Figure 1). The presence of a chiral carbon in propranolol is crucial for its activity[38]. Additionally, propranolol has a chiral carbon in its structure [39].
Figure 1: Propranolol.
The very essential note is the chiral carbon which present in the propranolol [40].
Increasing the binding affinity of VEGFR-2 inhibitors by extending their hydrophobic interaction with the active site: Design, synthesis, and biological evaluation of 1-(4-((1H-pyrazolo[3,4-d]pyrimidin-4-yl)oxy)phenyl)-3-(4-(trifluoromethyl)phenyl)urea derivatives as VEGFR-2 inhibitors was achieved by optimizing the hydrophobic interactions within the active site of VEGFR-2, resulting in compounds with improved binding affinity and potent biological activity [41].
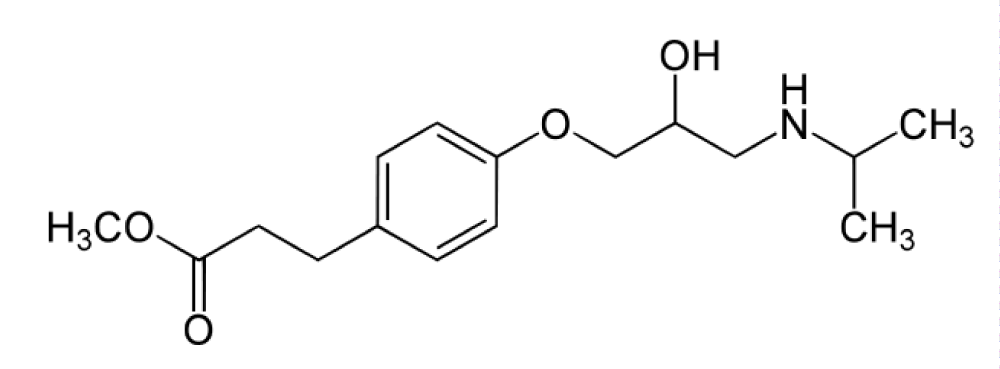
Atenolol
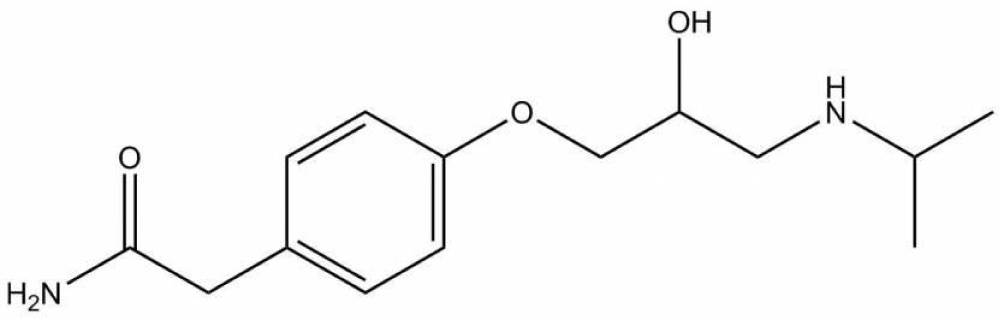
Atenolol is a β1-blocker used in the treatment of hypertension, which is raised from the dysfunction of the heart, so used in high blood pressure and heart-associated chest pain. Atenolol has phenoxy group chiral carbon and isopropyl on the amino group, which are characterized sharing of beta blockers, we note the only difference naphthyloxy is substituted by phenoxy (Figure 2).
Figure 2: Atenolol.
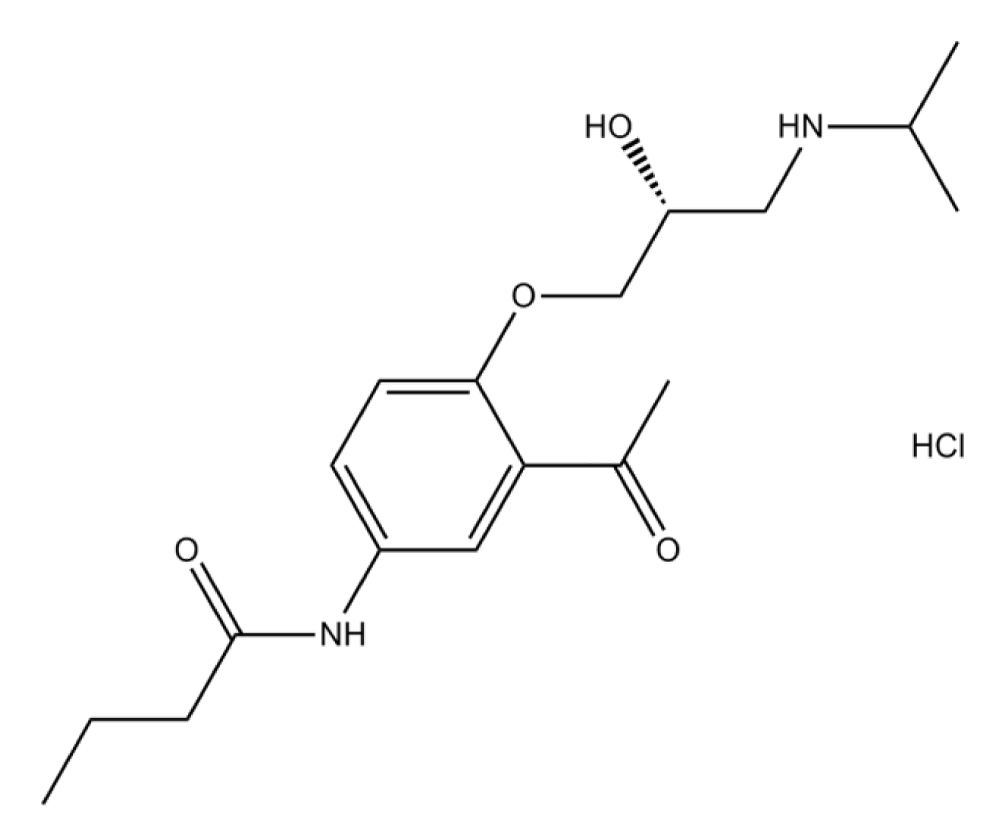
Bisoprolol
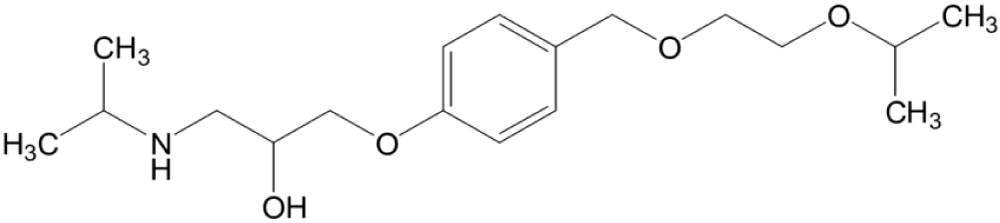
Bisoprolol is a β1 blocker that is used in heart diseases (hypertension) and tachyarrhythmia. The chemical structure of atenolol (β1 blocker) has a phenoxy group and isopropyl group on the amino group attached to chiral carbon (Figure 3) [42].
Figure 3: Bisoprolol.
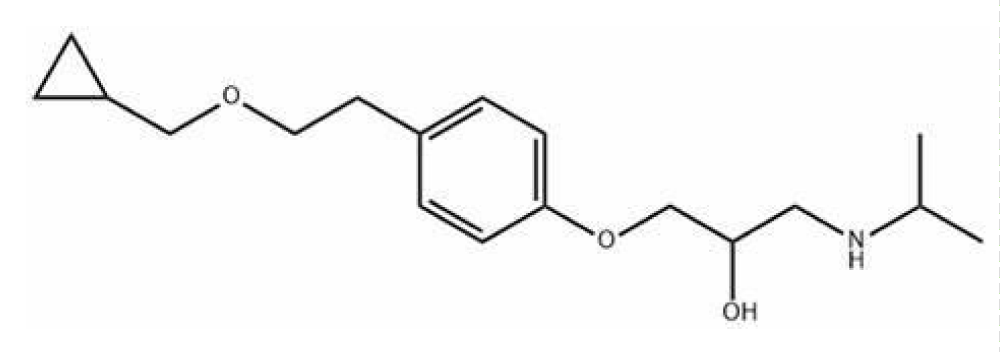
Nebivolol
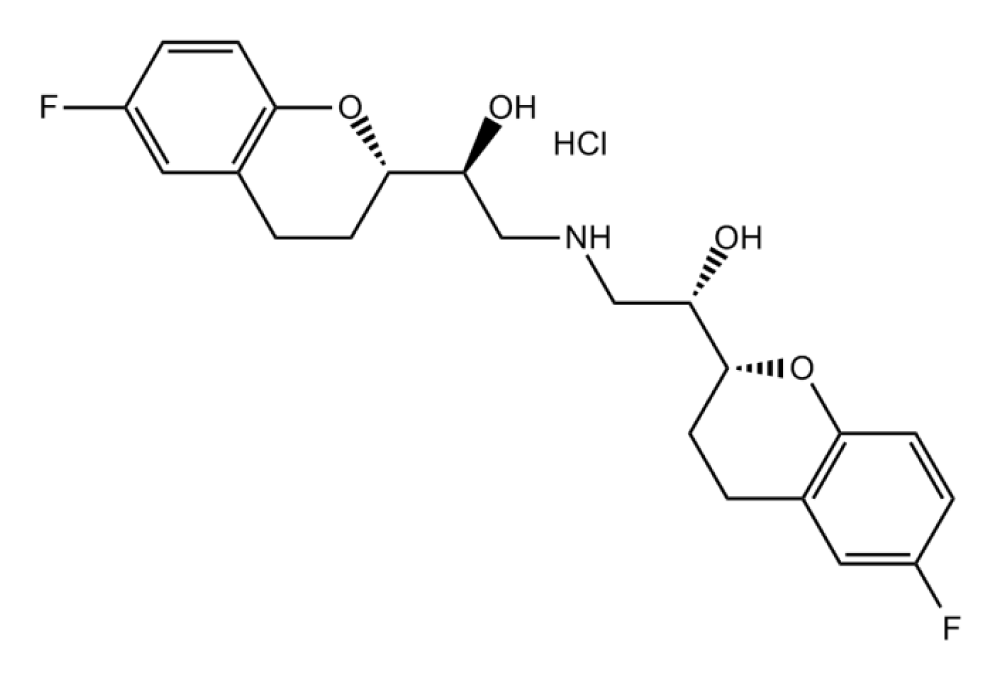
Nebivolol is a Beta1 blocker used in heart failure (hypertension), acts on beta receptors that are present in cardiac muscle, it is used orally only or in combination with another drug (Hydrochlorothiazide).
The nebivolol has two chiral carbons, and two phenoxy groups (which formed lactone) and the amino group becomes disubstituted (Figure 4) [43].
Figure 4: Nebivolol.
Metoprolol
Metoprolol is a drug that acts as a β1 blocker which is used in the management of high blood pressure accompanied by chest pain and fast heart rate in case of poor blood flow to the heart, it is used to prevent myocardial infarction and headaches (migraine). Metoprolol is a phenoxy derivative compound that has isopropyl on the amino group (monosubstituted amine) (Figure 5).
Figure 5: Metoprolol.
It is used in intravenous or oral administration.
β 1 blocker drug is a cardioselective drug characterized by rapid action and its action is very short duration, it differs from the previous compounds because it has no significant intrinsic sympathomimetic and membrane stabilizing activity, the esmolol has a phenoxy group, chiral carbon, and isopropyl group which they characterized beta-blocker activity (specific β 1) (Figure 6).
Figure 6: Esmolol.
Acebutolol
Acebutolol is a chemical structure that has β 1 blocker properties used in the treatment of hypertension and arrhythmias, it is a cardio-selective and has intrinsic sympathetic activity, as well as, it is used commonly in the treatment of angina. As with previous compounds, acebutolol has a phenoxy group, alkyl isopropyl group, and chiral center (Figure 7) [44].
Figure 7: Acebutolol.
Synthesis, molecular modeling, and anticonvulsant activity of benzoxazole derivatives provide insights into the development of novel agents for treating neurological disorders[45].
Betaxolol
It is a selective β 1 receptor blocker used in the treatment of glaucoma and hypertension and has no partial agonist activity and membrane stabilizing properties. Betaxolol structure includes a phenoxy group, chiral carbon center, and alkyl isopropyl on the amino group (Figure 8) [46]. Studies on the synthesis, modeling, and anticonvulsant activity of some phthalazinone derivatives further support the significance of stereoselectivity in drug development [47]. Furthermore, the development of novel compounds with anti-inflammatory properties, such as benzimidazole derivatives, has shown significant potential in treating various conditions due to their proton pump inhibitor activity [48].
Figure 8: Betaxolol.
Additionally, studies have demonstrated that anilides based on the 5, 5-diphenylimidazolidine-2, 4-dione scaffold exhibit significant anti-inflammatory, analgesic, and COX-1/2 inhibition activities, as supported by molecular docking studies [49].
Betaxolol is a selective β1 receptor blocker used in the treatment of glaucoma and hypertension and has no partial agonist activity and membrane stabilizing properties (Development of novel benzofuran-isatin conjugates as potential antiproliferative agents with the apoptosis-inducing mechanism in Colon cancer [50]. Betaxolol’s structure includes a phenoxy group, chiral carbon center, and alkyl isopropyl on the amino group [51].
In conclusion, the β1 receptor plays a crucial role in the cardiovascular system, specifically in regulating the heartbeat and increasing the pumping of blood from the heart. Various β blockers, such as propranolol, atenolol, bisoprolol, nebivolol, metoprolol, esmolol, acebutolol, and betaxolol, have been developed to target this receptor. These drugs exhibit stereoselectivity due to the presence of a chiral carbon in their chemical structure [48].
Propranolol, a non-selective β blocker, is used for the treatment of hypertension, irregular heartbeat, thyrotoxicosis, essential tremors, and other cardiovascular conditions. Atenolol, on the other hand, is a selective β1 blocker primarily used for hypertension and chest pain associated with heart conditions. Bisoprolol and nebivolol are also β1 blockers used in the management of heart diseases and hypertension.
Metoprolol is another β1 blocker that is effective in controlling high blood pressure, chest pain, and fast heart rates. Esmolol, a cardioselective β1 blocker, acts rapidly but has a short duration of action. Acebutolol, with its intrinsic sympathomimetic activity, is commonly prescribed for hypertension, arrhythmias, and angina. Lastly, betaxolol, a selective β1 receptor blocker, is used for the treatment of glaucoma and hypertension.
The development of these β1 blockers has significantly contributed to the management of various cardiovascular conditions. Their distinct chemical structures and stereoselectivity enable them to selectively target the β1 receptor, leading to improved therapeutic outcomes. Further research and development in this field, such as the development of 2-oxindolin-3-ylidene-indole-3-carbohydrazide derivatives as novel apoptotic and anti-proliferative agents toward colorectal cancer cells is an area of active research [52]. Further advancements in this field may reveal additional β1 receptor agonists with improved efficacy and reduced side effects, expanding treatment options for patients with cardiovascular disorders. Additionally, the ongoing research and evaluation of novel anti-cancer compounds remain a significant focus, promising potential advancements in cancer therapy [36] and may uncover additional β1 receptor agonists with enhanced efficacy and fewer side effects, providing even more options for patients with cardiovascular disorders.
- Ayyad RR, Mansour AM, Nejm AM, Hassan YAA, Ayyad AR. Stereo Selectivity of Histaminic Receptors Plays an Important Role in Anti-histaminic Activity. Curr Res Med Sci. 2024;3(1):10-17. Available from: https://www.pioneerpublisher.com/crms/article/view/634
- Al-Warhi T, El Kerdawy AM, Aljaeed N, Ismael OE, Ayyad RR, Eldehna WM, et al. Synthesis, Biological Evaluation and In Silico Studies of Certain Oxindole-Indole Conjugates as Anticancer CDK Inhibitors. Molecules. 2020;25(9):2031. Available from: https://pubmed.ncbi.nlm.nih.gov/32349307/
- Ayyad RR, Nejm AM, Ayyad AR. The Isomers of Some Drugs One Effective and the Other Is Toxic or Ineffective. Curr Res Med Sci. 2023;2(2):58-62 . Available from: https://www.pioneerpublisher.com/crms/article/view/317
- Alanazi AM, Abdel-Aziz AA, Shawer TZ, Ayyad RR, Al-Obaid AM, et al. Synthesis, antitumor and antimicrobial activity of some new 6-methyl-3-phenyl-4(3H)-quinazolinone analogues: in silico studies. J Enzyme Inhib Med Chem. 2016;31(5):721-35. Available from: https://pubmed.ncbi.nlm.nih.gov/26162029/
- El-Azab AS, Abdel-Aziz AA, Ayyad RR, Ceruso M, Supuran CT. Inhibition of carbonic anhydrase isoforms I, II, IV, VII and XII with carboxylates and sulfonamides incorporating phthalimide/phthalic anhydride scaffolds. Bioorg Med Chem. 2016;24(1):20-5. Available from: https://pubmed.ncbi.nlm.nih.gov/26678172/
- Ibrahim MK, Abd-Elrahman AA, Ayyad RRA, El-Adl K, Mansour AM, et al. Design and synthesis of some novel 2-(3-methyl-2-oxoquinoxalin-1 (2H)-yl)-N-(4-(substituted) phenyl) acetamide derivatives for biological evaluation as anticonvulsant agents. Bull Fac Pharm Cairo Univ. 2013;51(1):101-111. Available from: https://www.sciencedirect.com/science/article/pii/S1110093112000555
- Al Ward M, Abdallah AE, Zayed M, Ayyad R, El-Zahabi M. New immunomodulatory anticancer quinazolinone based thalidomide analogs: Design, synthesis and biological evaluation. 2023. Available from: https://assets-eu.researchsquare.com/files/rs-2916749/v1_covered_2c995cf5-39ef-46f0-a9b9-f0cb20f6d8c1.pdf?c=1686750506
- Mohamed MA, Ayyad RR, Shawer TZ, Abdel-Aziz AA, El-Azab AS. Synthesis and antitumor evaluation of trimethoxyanilides based on 4(3H)-quinazolinone scaffolds. Eur J Med Chem. 2016;112:106-113. Available from: https://pubmed.ncbi.nlm.nih.gov/26890117/
- Osman IA, Ayyad RR, Mahdy HA. New pyrimidine-5-carbonitrile derivatives as EGFR inhibitors with anticancer and apoptotic activities: design, molecular modeling and synthesis. New J Chem. 2022;46(24):11812-11827. Available from: https://pubs.rsc.org/en/content/articlelanding/2022/nj/d2nj01451c
- Mahdy H, Shaat M. Recent advances in drugs targeting protein kinases for cancer therapy. Al-Azhar J Pharm Sci. 2022;66(2):56-86. Available from: https://journals.ekb.eg/article_268384.html
- El-Adl K, El-Helby AA, Ayyad RR, Mahdy HA, Khalifa MM, Elnagar HA, et al. Design, synthesis, and anti-proliferative evaluation of new quinazolin-4(3H)-ones as potential VEGFR-2 inhibitors. Bioorg Med Chem. 2021;29:115872. Available from: https://pubmed.ncbi.nlm.nih.gov/33214036/ .
- Nassar E, El-Badry YA, Eltoukhy AMM, Ayyad RR. Synthesis and Antiproliferative Activity of 1-(4-(1H-Indol-3-Yl)-6-(4-Methoxyphenyl) Pyrimidin-2-yl) Hydrazine and Its Pyrazolo Pyrimidine Derivatives. Med Chem (Los Angeles). 2016;6:224-233. Available from: https://www.hilarispublisher.com/open-access/synthesis-and-antiproliferative-activity-of-141hindol3yl64methoxyphenylpyrimidin2ylhydrazine-and-its-pyrazolo-pyrimidine-derivativ-2161-0444-1000350.pdf
- Ayyad RR, Nejm AM, Elbahat ELT, Elnagar AM, Aljazar MA. The Configuration of Some Hormonal Compounds Play an Important Role in Pharmacological Action (Agonist, Antagonist, Active, More Active). 2023;2(3):23-29. Available from: https://www.pioneerpublisher.com/jpeps/article/view/418
- Ibrahim A, Sakr HM, Ayyad RR, Khalifa MM. Design, Synthesis, In‐Vivo Anti‐Diabetic Activity, In‐Vitro α‐Glucosidase Inhibitory Activity and Molecular Docking Studies of Some Quinazolinone Derivatives. ChemistrySelect. 2022;7(14). Available from: https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/slct.202104590
- Sakr HM, Ayyad RR, Mahmoud K, Mansour AM, Ahmed G. Design, Synthesis of Analgesics and Anticancer of Some New Derivatives of Benzimidazole. Int J Org Chem. 2021;11(03):144-169. Available from: https://www.scirp.org/journal/paperinformation?paperid=112320
- Ayyad RR. Synthesis and Biological Evaluation of Novel Iodophthalazinedione Derivatives as Anticonvulsant Agents. Al-Azhar J Pharm Sci. 2012;45(1):1-13. Available from: https://ajps.journals.ekb.eg/article_7146.html
- Al-Warhi T, Almahli H, Maklad RM, Elsayed ZM, El Hassab MA, Alotaibi OJ, et al. 1-Benzyl-5-bromo-3-hydrazonoindolin-2-ones as Novel Anticancer Agents: Synthesis, Biological Evaluation and Molecular Modeling Insights. Molecules. 2023;28(7):3203. Available from: https://pubmed.ncbi.nlm.nih.gov/37049966/
- Salem M, Ayyad R, Sakr H. Design and Synthesis of Some New Oxadiazole Derivatives as Anticancer Agents. Int J Org Chem. 2022;12(02):64-74. Available from: https://www.scirp.org/journal/paperinformation?paperid=117052
- Ayyad R, Sakr H, Gaafer A. Design and Synthesis of New Compounds Derived from Phenyl Hydrazine and Different Aldehydes as Anticancer Agents. Int J Org Chem. 2022;12(1):28-39. Available from: https://www.scirp.org/journal/paperinformation?paperid=116252
- Ayyad RA, Sakr HM, El-Gamal KM. Design, Synthesis, Computer Modeling and Analgesic Activity of Some New Disubstituted Quinazolin-4(3H)-ones. Med Chem. 2016;6(5):299-305. Available from: https://www.hilarispublisher.com/open-access/design-synthesis-computer-modeling-and-analgesic-activity-of-some-newdisubstituted-quinazolin43hones-2161-0444-1000360.pdf
- Ayyad R. Synthesis and Anticonvulsant Activity of 6-Iodo Phthalazinedione Derivatives. Al-Azhar J Pharm Sci. 2014;50(2):43-54. Available from: https://ajps.journals.ekb.eg/article_6930.html
- Abdel-Aziz AA, El-Azab AS, Alanazi AM, Asiri YA, Al-Suwaidan IA, Maarouf AR, et al. Synthesis and potential antitumor activity of 7-(4-substituted piperazin-1-yl)-4-oxoquinolines based on ciprofloxacin and norfloxacin scaffolds: in silico studies. J Enzyme Inhib Med Chem. 2016;31(5):796-809. Available from: https://pubmed.ncbi.nlm.nih.gov/26226179/
- Khalifa MM, Sakr HM, Ibrahim A, Mansour AM, Ayyad RR. Design and synthesis of new benzylidene-quinazolinone hybrids as potential anti-diabetic agents: In vitro α-glucosidase inhibition, and docking studies. J Mol Struct. 2022;1250:131768. Available from: https://www.scirp.org/reference/referencespapers?referenceid=3205061
- Ibrahim MK, El-Helby AEA, Ghiaty AH, Biomy AH, Abd-El Rahman AA. Modeling, synthesis and antihyperglycemic activity of novel quinazolinones containing sulfonylurea. 2009;7. Available from: https://d1wqtxts1xzle7.cloudfront.net/52726405/Modeling_Synthesis_and_Antihyperglycemic20170420-23469-3pjbru-libre.pdf?1492732308=&response-content-disposition=inline%3B+filename%3DModeling_Synthesis_and_Antihyperglycemic.pdf&Expires=1720587812&Signature=Eesy4nCT18iIb1sXIbwIDzN6JUb2SwzZRX560TBRvBfccFMef82Iq5oYZnhX70qFoBIOfBeb8fcmcdoFdSgwReIvyuDWSmN4kRNJLEaTKlaO-8BfM4JVUCVg8Yupg2LFclJ3Agd4bQjdVY6Q6faK3SZe06mVXDfbnDK834i6N67JZwxU2Ar9E7CmqDP9Dvrwxfkx~~fykomeM-WoJdPBSZNWMcdwz5aBYuiF6zwfVAdO~srvzi~bg172Cr7cwLYO08zrvdqdwTXAePXP-oa5vpzLJzP3ObrC2GmRv3UoxclVdp7O5YE~POGodm1HGEoJ-eoMKrL3Aw71AoH-NGZAbw__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA
- Al Ward MMS, Abdallah AE, Zayed MF, Ayyad RR, El-Zahabi MA. Design, synthesis and biological evaluation of newly triazolo-quinoxaline based potential immunomodulatory anticancer molecules. J Mol Struct. 2024;1298:137041. Available from: https://ui.adsabs.harvard.edu/abs/2024JMoSt129837041W/abstract
- Al-Suwaidan IA, Abdel-Aziz AA, Shawer TZ, Ayyad RR, Alanazi AM, El-Morsy AM, Mohamed MA, Abdel-Aziz NI, El-Sayed MA, El-Azab AS. Synthesis, antitumor activity and molecular docking study of some novel 3-benzyl-4(3H)quinazolinone analogues. J Enzyme Inhib Med Chem. 2016;31(1):78-89. Available from: https://pubmed.ncbi.nlm.nih.gov/25815668/
- Ayyad RR, Nejm AM, Ayyad AR. The Activity of Some Antibiotics Depend on Stereochemistry of Them (Its Structure). J Prog Eng Phys Sci. 2023;2(2):5-7. Available from: https://www.pioneerpublisher.com/jpeps/article/view/230
- El-Helby AGA, Ayyad RR, Sakr HM, Abdelrahim AS, El-Adl K, Sherbiny FS, et al. Design, synthesis, molecular modeling, and biological evaluation of novel 2,3-dihydrophthalazine-1,4-dione derivatives as potential anticonvulsant agents. J Mol Struct. 2017;1130:333-351. Available from: https://www.sciencedirect.com/science/article/abs/pii/S0022286016310936
- Ayyad RR, Nejm AM, Abdelaleem YH, Ayyad AR. Hydrophobicity, Transport and Target Sites of Action Are Important for the Activity of Many Drugs. Curr Res Med Sci. 2023;2(3):15-19. Available from: https://www.pioneerpublisher.com/crms/article/view/375
- Sakr H, Ayyad RR, El-Helby AA, Khalifa MM, Mahdy HA. Discovery of novel triazolophthalazine derivatives as DNA intercalators and topoisomerase II inhibitors. Arch Pharm (Weinheim). 2021;354(6):e2000456. Available from: https://pubmed.ncbi.nlm.nih.gov/33554352/
- Eissa IH, Metwaly AM, Belal A, Mehany ABM, Ayyad RR, El-Adl K, et al. Discovery and antiproliferative evaluation of new quinoxalines as potential DNA intercalators and topoisomerase II inhibitors. Arch Pharm (Weinheim). 2019;352(11):e1900123. Available from: https://pubmed.ncbi.nlm.nih.gov/31463953/
- Ayyad RR, Nejm AM, Hassan YAA, Ayyad AR. The Lipid Solubility of Most Drugs Play Important Role of Its Pharmacological Action and Duration of Action. J Prog Eng Phys Sci. 2023;2(4):1-6. Available from: https://www.pioneerpublisher.com/jpeps/article/view/486
- El-Helby AA, Ayyad RRA, Zayed MF, Abulkhair HS, Elkady H, El-Adl K. Design, synthesis, in silico ADMET profile and GABA-A docking of novel phthalazines as potent anticonvulsants. Arch Pharm (Weinheim). 2019;352(5). Available from: https://pubmed.ncbi.nlm.nih.gov/30989729/
- Ayyad RR, Nejm AM, Hassan YAA, Ayyad AR. Mechanism of Action of Many Drugs Depend on Enzyme Inhibition. Curr Res Med Sci. 2023;2(4):1-9. Available from: https://www.pioneerpublisher.com/crms/article/view/494
- El-Adl K, El-Helby AGA, Sakr H, Ayyad RR, Mahdy HA, Nasser M, et al. Design, synthesis, molecular docking, anticancer evaluations, and in silico pharmacokinetic studies of novel 5-[(4-chloro/2,4-dichloro) benzylidene] thiazolidine-2,4-dione. Arch Pharm. 2021;354(2):2000279. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/ardp.202000279
- El-Helby AGA, Sakr H, Ayyad RRA, El-Adl K, Ali MM, Khedr F. Design, synthesis, in vitro anti-cancer activity, ADMET profile and molecular docking of novel triazolo [3,4-a] phthalazine derivatives targeting VEGFR-2 enzyme. Anti-Cancer Agents Med Chem. 2018;18(8):1184-1196. Available from: https://www.ingentaconnect.com/content/ben/acamc/2018/00000018/00000008/art00015
- Sakr H, Otify I, Ayyad RR, Elwan A. VEGFER-2 INHIBITORS AND QUINAZOLINE-BASED ANTICANCER AGENTS. Al-Azhar J Pharm Sci. 2022;68(2):111-129. Available from: https://ajps.journals.ekb.eg/article_332170.html
- Abdel-Aziz AA, Abou-Zeid LA, ElTahir KEH, Ayyad RR, El-Sayed MA, El-Azab AS. Synthesis, anti-inflammatory, analgesic, COX-1/2 inhibitory activities and molecular docking studies of substituted 2-mercapto-4(3H)-quinazolinones. Eur J Med Chem. 2016;121:410-421. Available from: https://pubmed.ncbi.nlm.nih.gov/27318118/
- El-Helby AGA, Ayyad RR, El-Adl K, Elwan A. Quinoxalin-2(1H)-one derived AMPA-receptor antagonists: Design, synthesis, molecular docking and anticonvulsant activity. Med Chem Res. 2017;26:2967-2984. Available from: https://link.springer.com/article/10.1007/s00044-017-1996-5
- Elhelby AA, Ayyad RR, Zayed MF. Synthesis and biological evaluation of some novel quinoxaline derivatives as anticonvulsant agents. Arzneimittelforschung. 2011;61(7):379-81. Available from: https://pubmed.ncbi.nlm.nih.gov/21899204/
- Eldehna WM, Abou-Seri SM, El Kerdawy AM, Ayyad RR, Hamdy AM, Ghabbour HA, et al. Increasing the binding affinity of VEGFR-2 inhibitors by extending their hydrophobic interaction with the active site: Design, synthesis and biological evaluation of 1-substituted-4-(4-methoxybenzyl)phthalazine derivatives. Eur J Med Chem. 2016 May 4;113:50-62. doi: 10.1016/j.ejmech.2016.02.029. PMID: 26922228. Available from: https://pubmed.ncbi.nlm.nih.gov/26922228/
- El-Helby AA, Ayyad RRA, El-Adl K, Elkady H. Phthalazine-1,4-dione derivatives as non-competitive AMPA receptor antagonists: design, synthesis, anticonvulsant evaluation, ADMET profile and molecular docking. Mol Divers. 2019 May;23(2):283-298. doi: 10.1007/s11030-018-9871-y. PMID: 30168051. Available from: https://pubmed.ncbi.nlm.nih.gov/30168051/
- El-Helby AA, Ayyad RRA, Sakr H, El-Adl K, Ali MM, Khedr F. Design, Synthesis, Molecular Docking, and Anticancer Activity of Phthalazine Derivatives as VEGFR-2 Inhibitors. Arch Pharm (Weinheim). 2017 Dec;350(12). doi: 10.1002/ardp.201700240. PMID: 29131379. Available from: https://pubmed.ncbi.nlm.nih.gov/29131379/
- El-Helby AA, Sakr H, Ayyad RR, Mahdy HA, Khalifa MM, Belal A, et al. Design, synthesis, molecular modeling, in vivo studies and anticancer activity evaluation of new phthalazine derivatives as potential DNA intercalators and topoisomerase II inhibitors. Bioorg Chem. 2020;103:104233. Available from: https://pubmed.ncbi.nlm.nih.gov/32882440/
- El-Helby AA, Ibrahim MK, Abdel-Rahman AA, Ayyad RRA, Menshawy MA, et al. Synthesis, molecular modeling, and anticonvulsant activity of benzoxazole derivatives. Al-Azhar J Pharm Sci. 2017;40:252-270.
- El-Helby AGA, Ayyad RRA, El-Adl K, Sakr H, Abd-Elrahman AA, Eissa IH, et al. Design, molecular docking, and synthesis of some novel 4-acetyl-1-substituted-3,4-dihydroquinoxalin-2(1H)-one derivatives for anticonvulsant evaluation as AMPA antagonists. Med Chem Res. 2016;25:3030-3046. Available from: https://www.infona.pl/resource/bwmeta1.element.springer-doi-10_1007-S00044-016-1723-7
- Ayyad RRA, Sakr H, El-Gamal K. Synthesis, modeling and anticonvulsant activity of some phthalazinone derivatives. Am J Org Chem. 2016;6(1):29-38. Available from: http://article.sapub.org/10.5923.j.ajoc.20160601.04.html
- Ayyad RR, Sakr HM, El-Gamal KM, Eissa IH, et al. Anti-Inflammatory, Proton Pump Inhibitor and Synthesis of Some New Benzimidazole Derivatives. Der Chemica Sinica. 2017;8(1):184-197. Available from: https://www.imedpub.com/articles/antiinflammatory-proton-pump-inhibitor-and-synthesis-of-some-newbenzimidazole-derivatives.pdf
- Abdel-Aziz AA, El-Azab AS, Abou-Zeid LA, ElTahir KE, Abdel-Aziz NI, Ayyad RR, Al-Obaid AM. Synthesis, anti-inflammatory, analgesic and COX-1/2 inhibition activities of anilides based on 5,5-diphenylimidazolidine-2,4-dione scaffold: Molecular docking studies. Eur J Med Chem. 2016;115:121-131. Available from: https://pubmed.ncbi.nlm.nih.gov/26999325/
- Eldehna WM, Salem R, Elsayed ZM, Al-Warhi T, Knany HR, Ayyad RR, et al. Development of novel benzofuran-isatin conjugates as potential antiproliferative agents with apoptosis inducing mechanism in Colon cancer. J Enzyme Inhib Med Chem. 2021;36(1):1424-1435. Available from: https://pubmed.ncbi.nlm.nih.gov/34176414/
- Zayed MF, Ayyad RR. Some novel anticonvulsant agents derived from phthalazinedione. Arzneimittelforschung. 2012;62(11):532-536. Available from: https://pubmed.ncbi.nlm.nih.gov/22956351/
- Eldehna WM, Abo-Ashour MF, Al-Warhi T, Al-Rashood ST, Alharbi A, Ayyad RR, et al. Development of 2-oindolin-3-ylidene-indole-3-carbohydrazide derivatives as novel apoptotic and anti-proliferative agents towards colorectal cancer cells. J Enzyme Inhib Med Chem. 2021;36(1):319-328. Available from: https://pubmed.ncbi.nlm.nih.gov/33345633/