More Information
Submitted: August 28, 2024 | Approved: September 02, 2024 | Published: September 03, 2024
How to cite this article: Luisetto M, Almukthar N, Edbey K, Mashori GR, Fiazza C, Dona’ l, et al. Oral Suspension as Versatile Galenic Formulation in Pediatry. Arch Pharm Pharma Sci. 2024; 8(1): 091-099. Available from: https://dx.doi.org/10.29328/journal.apps.1001062
DOI: 10.29328/journal.apps.1001062
Copyright License: © 2024 Luisetto M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction inany medium, provided the original work is properly cited.
Keywords: Pediatric galenic formula; Magistral; Oral suspension; Chemico-physical property shortcomings; API; Excipients; Ready-for-use vehicle; Innovations
Oral Suspension as Versatile Galenic Formulation in Pediatry
Mauro Luisetto1*, Almukthar N2, Edbey K3, Mashori GR4, Fiazza C5, Dona’ l6, Cabianca L7 and Latyshev O8
1Independent Researcher Applied Pharmacologist, Hospital Pharmacist Manager, IMA Academy, Marijnskaya, PC Area, 29121, Italy
2Professor, Physiology, College of Medicine, University of Babylon, Hilla, Iraq
3Professor of Physical Chemistry, Libyan Authority for Scientific Research, Libya
4Professor, Department of Medical & Health Sciences for Women, Peoples University of Medical and Health Sciences for Women, Pakistan
5Medical Pharmacologist, Hospital Pharmacist Manager, Independent Researcher, Italy
6Independent Researcher, Hospital Pharmacist, Italy
7Medical Laboratory Turin, Citta della salute, Italy
8YU President, IMA Academy, Russia
*Address for Correspondence: Mauro Luisetto, Independent Researcher Applied Pharmacologist, Hospital Pharmacist Manager, IMA Academy, Marijnskaya, PC Area, 29121, Italy, Email: [email protected]
In the last years, there has been an increase in the prescription of drugs in pediatry as a pharmaceutical form of oral suspension. The same is true in commerce there are various producers that provide specific ready-for-use excipients to make more easier to prepare OS in the galenic laboratory. The aim of this work is to verify the advantages of this pharmaceutical form to cover pediatric dosages vs. other forms and also to overcome shortcomings of some crucial registered drugs. In this work scientific literature is reported that also relates to some ready-for-use products as bases-vehicle for suspension and some formulations of interest
Oral suspensions (OS) are liquid pharmaceutical forms and are used when there is not a suitable solvent available to dissolve a specific drug. Such as when the Active pharmaceutical ingredients (APIs) in solutions show reduced stability, when a more gradual absorption of the API vs. solutions is needed, and when an API is insoluble in water with suspension and is possible to reach more higher concentration than the solutions.
OS is generally more stable than oral solution for example for antibiotics. The OS can mask the taste of the drugs and have controlled release of the API. Other uses are in the shortcomings of registered drugs or due to the instability problem of the API. The same oral suspension can be an alternative to solid oral form like CPS or CP (easier to administrate in pediatry, better swallowing).
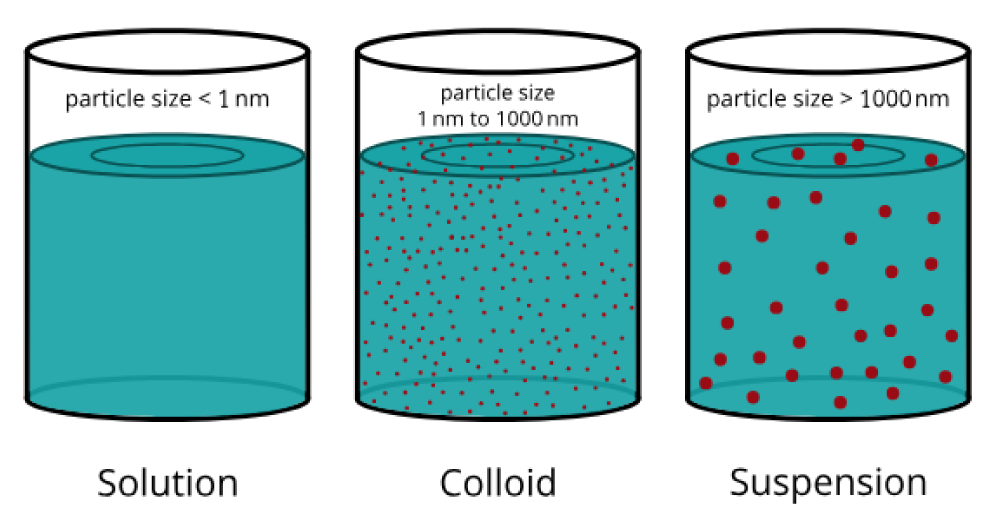
A disperse system is a two-phase system in which an insoluble phase (solid particles, or liquid droplets) is distributed through a continuous phase. The solids particles are not all of the same size. This is the dispersion of solids into a liquid, with a diameter of about 0.5-1 µm, a 100µm, and insoluble or partially soluble into the dispersant medium. The dispersant phase can be aqueous (for internal use) or oleos (for external use) (Figure 1).
Figure 1: Solutions/suspension particle size.
The solid particle can have affinity or not with the liquid. The lyophilic ones with affinity can produce stable suspension with easy re-dispersion after sedimentation. Instead the lyophobic produces unstable suspensions (that need tensioactives to reduce the interface tension). The tensioactive used are adsorbed around the solid particle producing a monolayer between the two phases and facilitating the production of a suspension (wetting property).
Generally, viscose vehicles are used to reduce the sedimentation viscosity. In normal galenic pharmacy practice, API or pharmaceutic powder or for example, CP or CPS are used. API in suspension shows a higher rate of bioavailability than other pharmaceutical forms: Solution> oral suspension>cps, >tablets > coated tablets. Related to the disadvantages of physical stability and sedimentation.
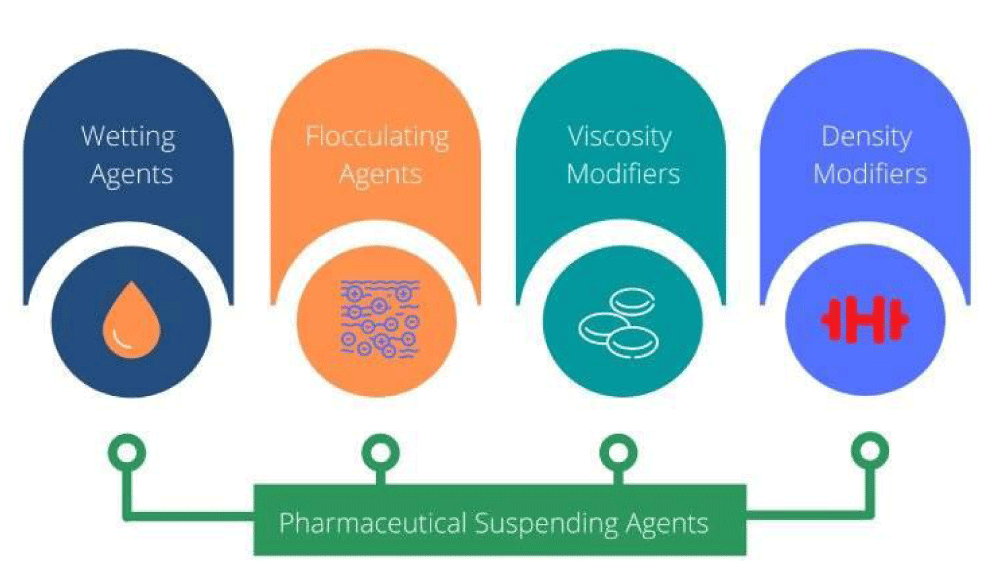
The solid particles are interested in attractive forces like Van der Waals or repulsive- ions or electrical charges on their surface. Between the formulation of interest are the suspending agents used as excipients [1] (Figure 2).
Figure 2: Pharmaceutical Suspending Agents [1].
Suspending agents: Hydrophilic colloids that produce colloid dispersions, they act also on the viscosity. For example, sodium alginate, methylcellulose 1-2%, hydroxyethylcellulose, hydroxypropyl cellulose, and hydroxypropylmethylcellulose.
Wetting agents: These are surface active agents, hydrophilic colloids, and solvents.
Flocculating agents: Such as electrolytes, ionic surfactants, and polymer flocculating agents. (Starches, alginates, cellulose ethers, tragacanth gum, carbomers, and aluminium silicate clays).
Viscosity modifiers: These are polysaccharides like acacia, tragacanth gum, sodium alginate, starch and starch derivatives, xanthan gum, and pectin.
Water-soluble cellulose ethers: To increase the viscosity of aqueous systems in which they are dispersed like methylcellulose hydroxyethylcellulose, sodium carboxymethylcellulose, and microcrystalline cellulose, and Hypromellose. The viscosity-increasing properties of cellulose ethers depend on the molecular weight and degree level of substitution.
Hydrated silicates are naturally occurring siliceous clays that exist as colloids in water like Bentonite, and Magnesium aluminium silicate. Carbomers are high molecular weight cross-linked polyacrylic acid polymers that swell in water to produce viscous hydrogels depending on the degree of cross-linking.
Density modifiers: From Stokes’ law, it is clear that if the densities dispersed and dispersing medium are of the same magnitude sedimentation would be significantly slowed down. So changing the density of the dispersing medium, for example, the addition of glycerol, propylene glycol, polyethylene glycol, or sucrose-based syrups, can significantly modify densities and be leveraged to control the instability.
Others:Electrolytes like aluminium closure, where alum can induce a negative charge on the surface of the solid particle increasing the repulsion force.
Buffers to control the pH variation.
Preservatives for microbiological needs (nipagin, potassium sorbate, and alcohol).
Cosolvents like glycerol, sorbitol, propyleneglycol.
For the characteristics of the various excipients used, see the Handbook of Pharmaceutical Excipients latest version [2].
Stability of oral suspension
Physical stability: Factors like appearance, odour, taste, pH, specific gravity, sedimentation, ZETA potential, compatibility with container, microscopic examination, cristal size, and uniform drug distribution test.
Chemical stability: Factors like API degradation, change in viscosity, antimicrobial property, incompatibility with the preservant, degradation of the preservant, and adsorbion of the preservant on the API.
Because often with water liquid formulations there is a risk of microbial contamination. The preservant agent used must be compatible with the rest and API without interference with the stability of the suspension. Because the specific weight is higher than the water phase it is clear that the particle will deposit, which is also related to size and other factors. The sediment is tolerable if easily re-dispersible. If it becomes compact or cake, with particles linked between them in various ways, this particle will be re-dispersed in difficult ways producing aggregates with compromised disponibility of the API. In magistral galenic, the need to mix before use contributes to the reduce this phenomenon. Instead in industrial products stored for a long time, this risk can happen. To avoid this, specific vehicles with various excipients are used.
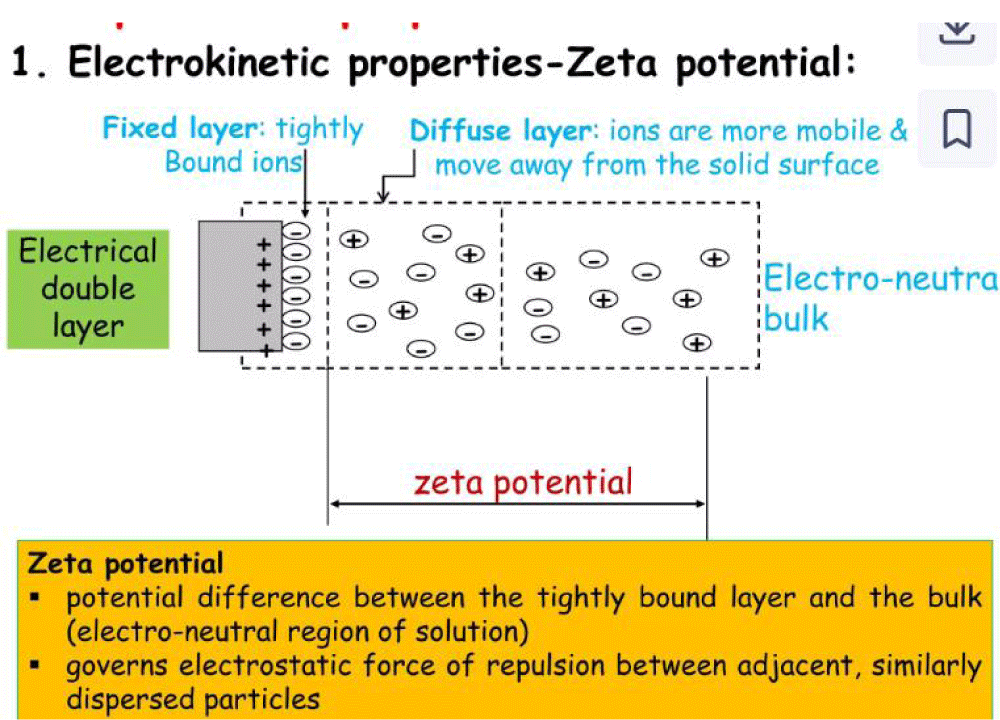
Between physical factors that influence the stability, it is possible to see that particle size, viscosity, electrical charge, suspensoid concentration, wetting agent, and surfactant use are among them. According to Stokes’ law, sedimentation velocity is directly proportional to the square of the particle diameter (i.e., too small or too large particles must be avoided). Differences in density between the API and the suspension agents, like if the API has a much lower density than the vehicle, it can float and cannot produce a good oral suspension. The Brownian movement makes it possible to balance the gravity of the solid particle in the medium. According to the ZETA potential related to the particle charge, if the attractive force exceeds the repulsive force, flocculation can happen. In a deflocculated system instead, the repulsion forces are greater than the attraction. Flocculating agents can be electrolytes like NaCl and phosphate salts, tensioactives, gum, and soluble cellulose derivates (Figures 3,4).
Figure 3: The ZETA potential. From Hanieh.
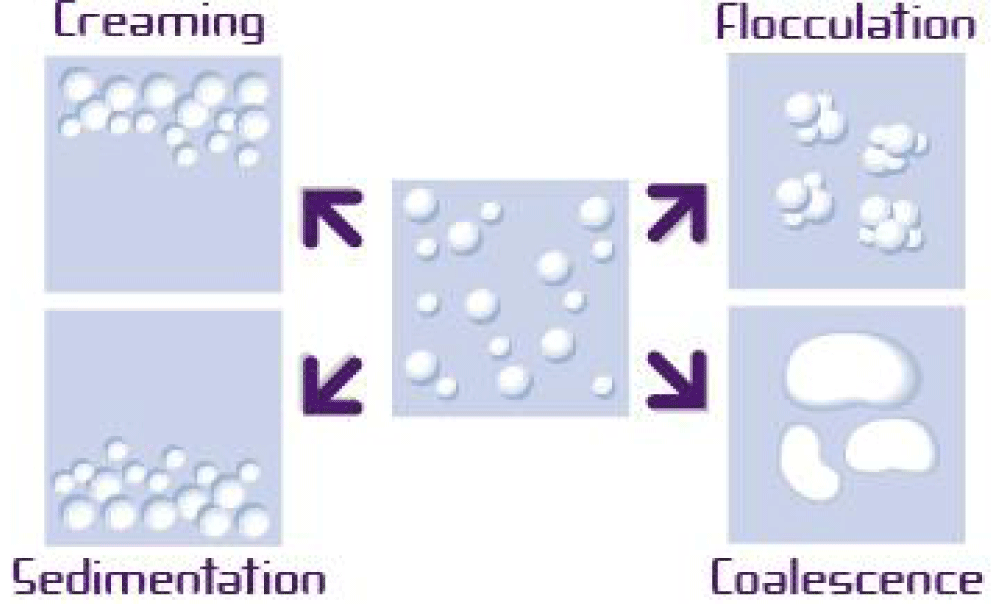
Figure 4: Destabilization process of liquid dispersion.
Floculated suspensions are formed flocculi with liquid above the sediment re-dispersible using light shaking. Deflocculated suspension is where the particles settled on the bottom are linked more between them (cake), and it is difficult to resuspend (Figure 5).
Figure 5: SUSPENSION deflocculated/flocculated. From Sahib.
According to the Italian normative rules, Law 30 December 2023, n. 214, article 16, “Preparation of galenic drugs”, allows the use of pharmaceutical industry API in the magistral formula. And in a ruling of the Council of State, (Section III, n. 4257/2015), the legitimacy of the packaging of industrial drugs in order to use the API in preparing a magistral formula was recognized-if not possible to proceed in other ways, but clarifying that “it is needed to turn directly to the holder of the patent or to the distributor of the related drug”.
According to Samz Morg in the “Enhancing Pediatric Care: The Importance of Oral Suspensions in Pediatric Drug Delivery” article in 2024, “Pediatric oral suspensions are liquid dosage forms consisting of finely divided drug particles suspended in a liquid vehicle, typically water with additives such as suspending agents, flavoring agents, and sweeteners. Unlike solutions, which are homogeneous mixtures of drug molecules dissolved in a liquid, suspensions contain insoluble drug particles that settle over time and require shaking before administration to ensure uniform drug distribution” [3].
Related to the control of technical pharmaceuticals for oral suspension, it is important to check on the suspension, granulometry, sedimentation, re-dispersibility, viscosity, density accelerated aging, and the API title. Required by pharmacopeia in the galenic lab is to verify the right procedure followed, check the aspect of re-dispersibility of the phases, and check the packaging and its seal-right labeling also with conservation condition.
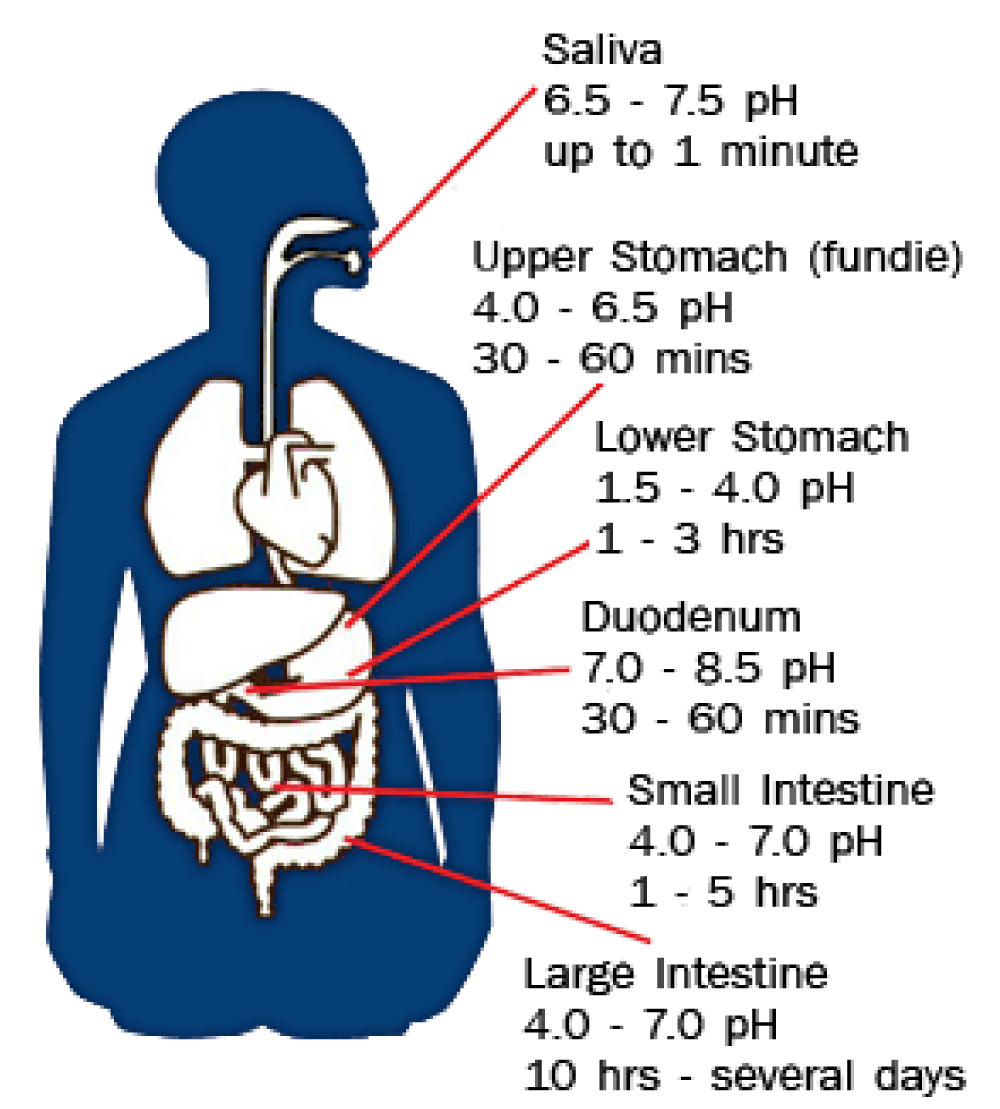
However, for this kind of galenic preparation, it is crucial to also consider the pH of the GI tract and its variation. As quoted from J Fallingborg’s work in 1999, “The intraluminal pH is rapidly changed from highly acid in the stomach to about pH 6 in the duodenum. The pH gradually increases in the small intestine from pH 6 to about pH 7.4 in the terminal ileum. The pH drops to 5.7 in the caecum, but again gradually increases, reaching pH 6.7 in the rectum” [4] (Figure 6).
Figure 6: GI PH variation [5].
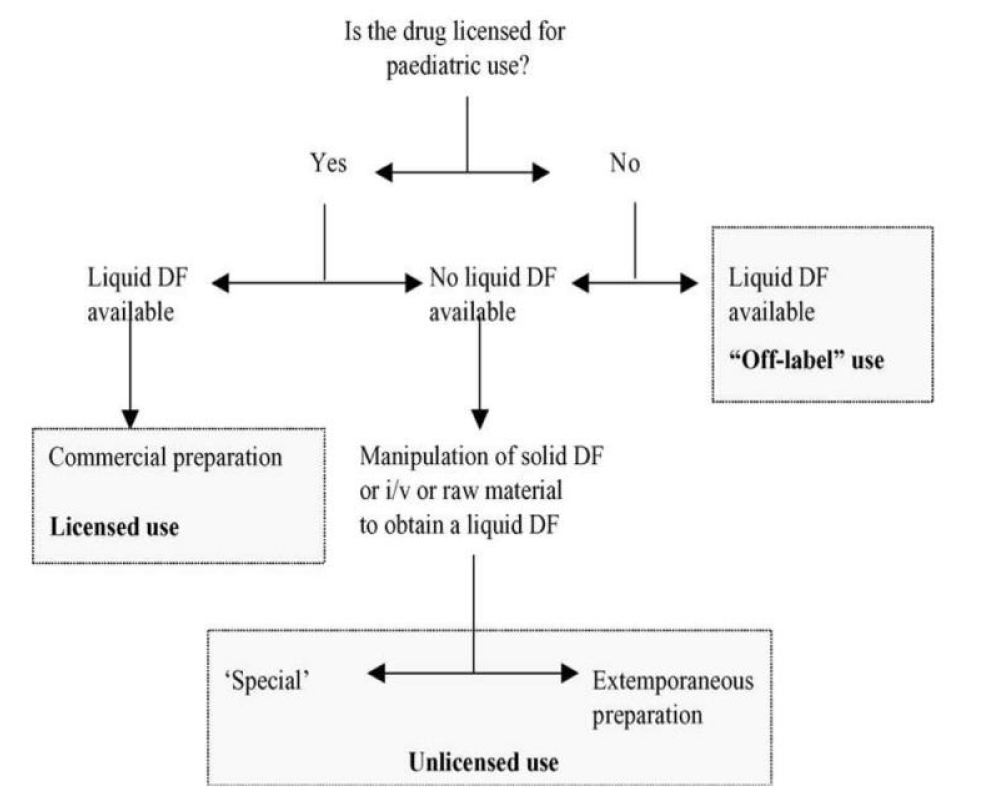
Figure 7: Decision pathway for providing oral doses to children for whom whole tablets/capsules are unsuitable [6].
The API can be in fact gastro sensible to the gastric acid fluids.
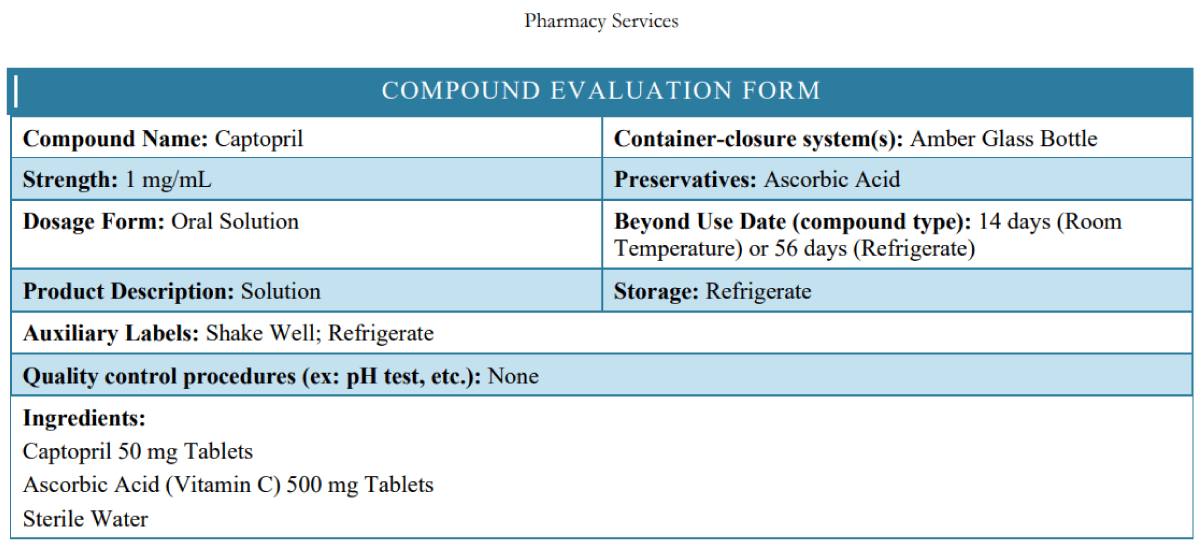
From the US PHARMACIST (8) for the Captopril 1 mg/mL Oral Solution, “Stability: The USP default beyond-use date for preserved aqueous oral liquids is 35 days. However, according to captopril stability studies, this formulation is stable for 14 days at controlled room temperature and for 56 days when refrigerated” (Figure 8).
Figure 8: Captopril 1 mg/ml evaluation form from the Nationwide Children’s Hospital (using preservative: ascorbic acid) [7].
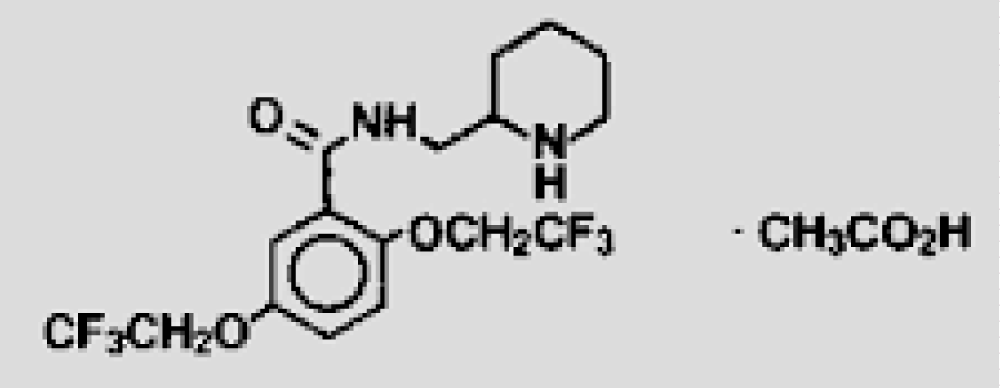
According to Matthew Grissinger in the “Life-Threatening Errors With Flecainide Suspension in Children” article in 2018 [9], it states that “Because it is available commercially only as 50-mg, 100-mg, and 150-mg tablets, it must be compounded into a suspension when needed for infants and small children. Unfortunately, errors during preparation and dosing of the suspension have occasionally led to serious overdoses that resulted in cardiac emergencies and required immediate therapeutic intervention. Overdoses can lead to seizures and cardiotoxicity, including ventricular tachycardia and fibrillation due to sodium-channel blockade. In hospitals, pharmacy labels should specify the dose in terms of both mg and mL, followed by the concentration, such as “Flecainide 5 mg (0.25 mL) 20 mg/mL suspension” (Figure 9).
Figure 9: Flecainide acetate formula.
Propranolol oral suspension 5mg/ml. Procedure from Sickkids [10] (Figure 10):
Figure 10: Beyond-used date (BUD): 91 days at room temperature [10].
Equipment: It is needed mortar and pestle glass stirring rod, graduated measure
Procedure:
1. Following Department procedures for risk assessment/training/PPE/equipment/facilities/NAPRA level;Crush tablets in the mortar to a fine powder with a pestle, or, soak tablets in a small amount of vehicle for at least 30 minutes.
2. Add a small amount of vehicle to powder and levigate to a smooth paste with a pestle. If soaked tablets, then levigate tablets into a smooth paste with a pestle. Continue to levigate as the vehicle is added in small amounts until a liquid is formed.
3. Transfer liquid contents from mortar to graduate.
4. Use a small amount of vehicle to rinse mortar and add it to graduate.
5. Use the vehicle to q.s. to the final volume. Stir well. It will be a very chunky suspension.
6. Transfer to an amber bottle and label.
7. Let suspension sit for 2 hours - 3 hours with intermittent shaking before using. Viscous chunks will fully dissolve into a smooth suspension.
From Preparation of extemporaneous oral liquid in the hospital pharmacy by Márcio Robert Mattos da Silva, Letícia Pereira Dysars, Elisabete Pereira dos Santos, and Eduardo Ricci Júnior in 2020 [11], “The stability of an extemporaneous suspension of 1.5 mg/mL propafenone was determined. To prepare the suspension, 150 mg of propafenone tablet was triturated to a fine powder in a mortar, and then 100 mL of pomegranate syrup was added. The suspension was placed in an amber glass flask, where one flask was stored at 3 °C - 5 °C and the other at 15 ± 5 °C. In both storage conditions, the suspension remained stable for 90 days”.
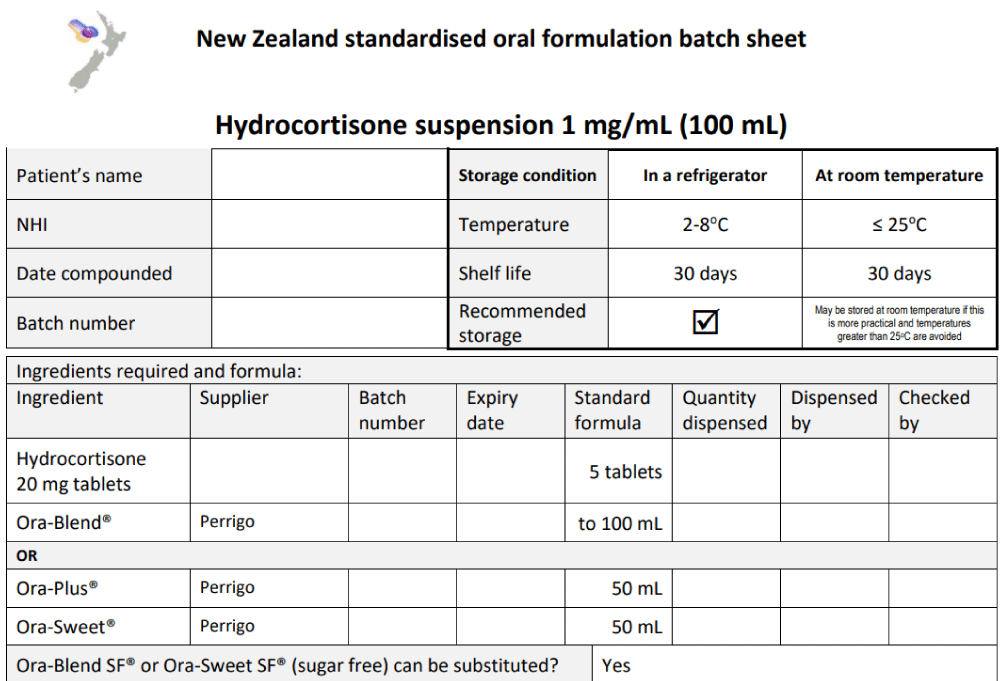
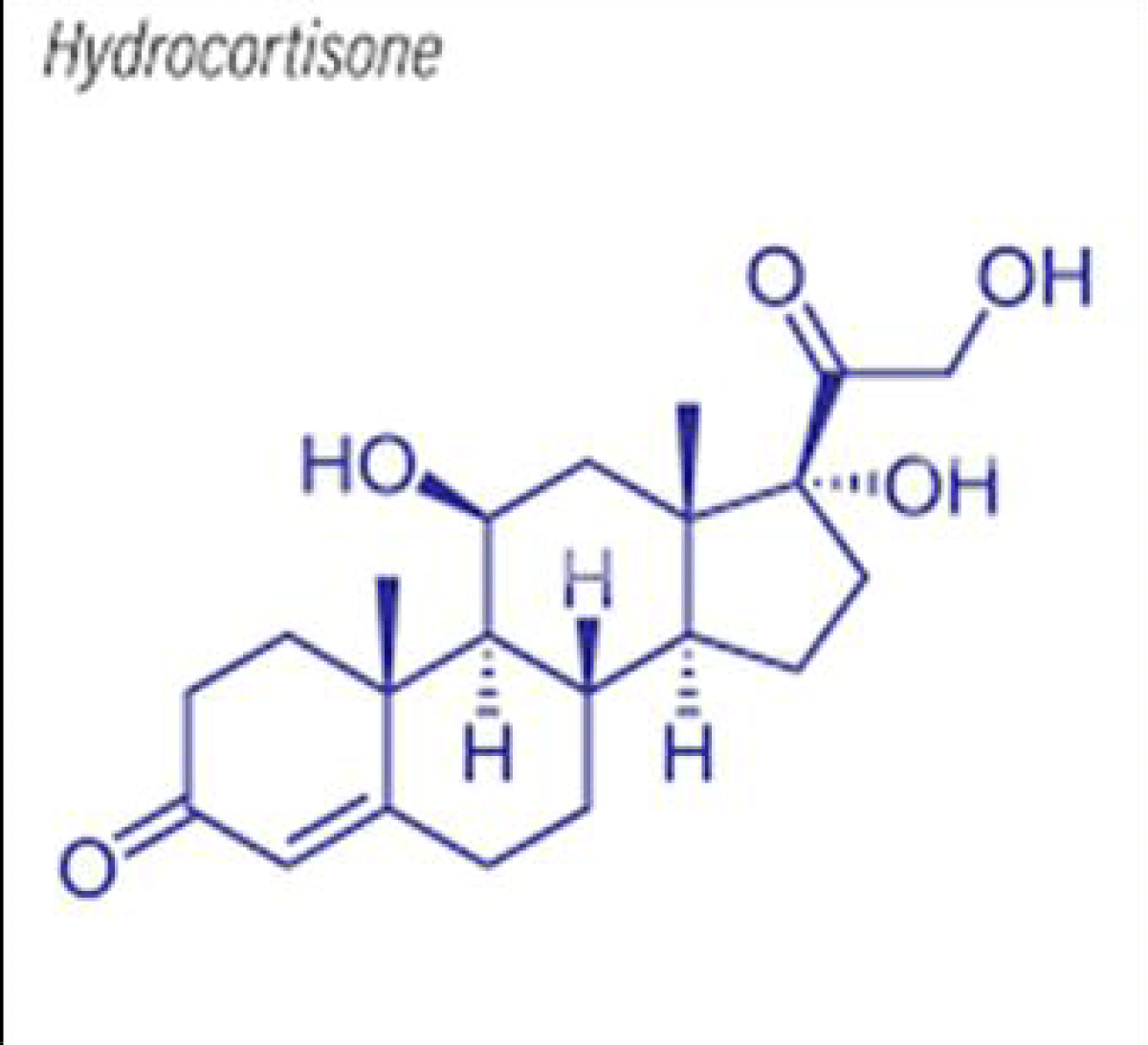
Other preparations such as sildenafil citrate oral solution and hydrocortisone suspension are given as examples [12,13] (Figures 11,12]. The hydrocortisone structural formula is shown in Figure 13.
Figure 11: Sildenafil oral liquid 2,5 mg/ml formulation by the US PHARMACIST [12].
Figure 12: New Zealand standardised oral formulation batch sheet-Hydrocortisone suspension 1 mg/mL (100 mL) from the Pharmaceutical Society of New Zealand (Special instructions: 1. Shake well before use, and 2. Store in a refrigerator) [13].
Figure 13: Structural formula of Hydrocortisone.
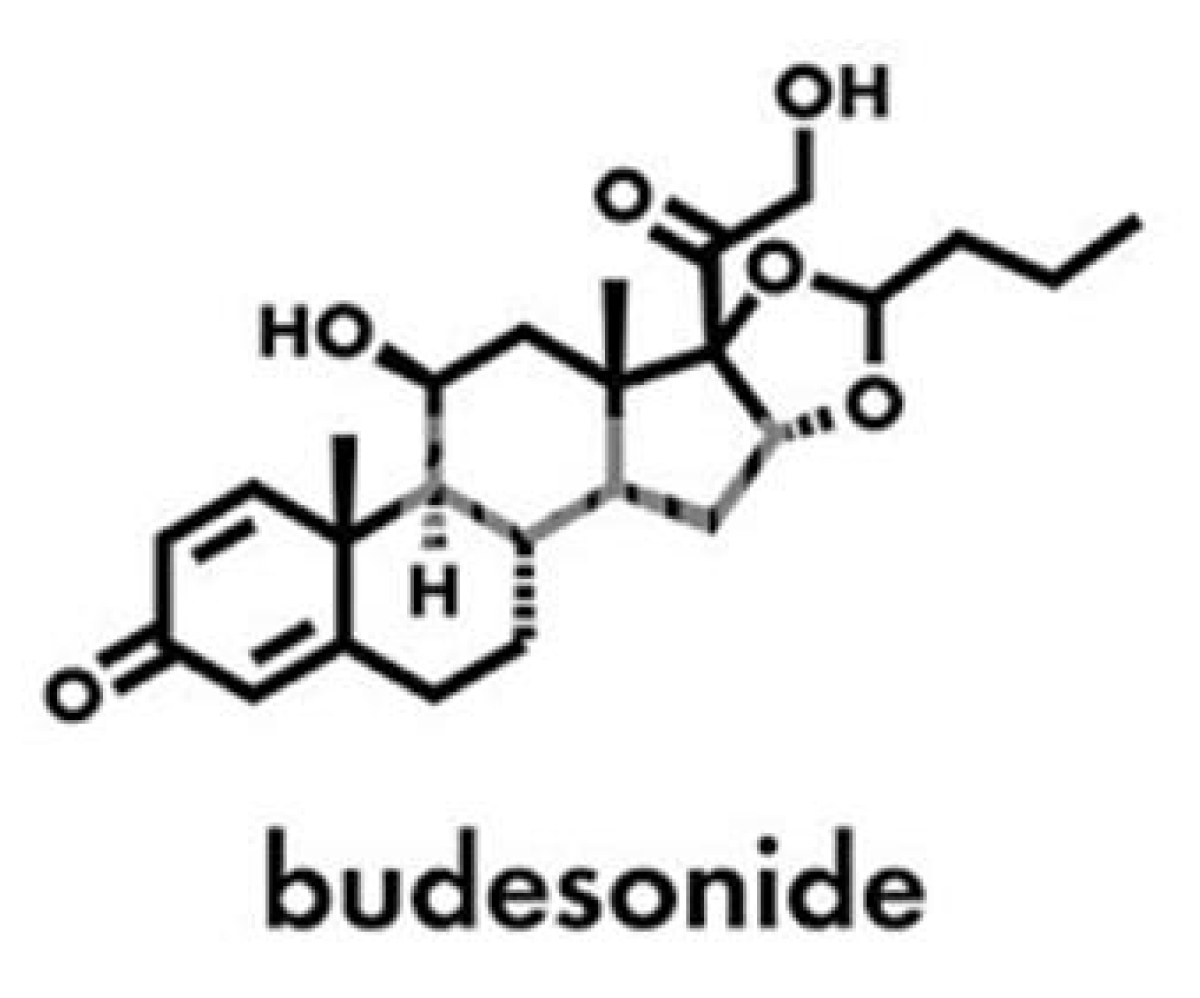
Budesonide (Figure 14) 2 mg/10 ml Oral Suspension is a homogeneous liquid medication, formulated to treat conditions such as asthma, allergic rhinitis, Crohn’s disease, ulcerative colitis, and eosinophilic esophagitis.
Figure 14: Budesonide structure.
Budesonide oral suspension for Eosinophilic esophagitis: An example of formulation Budesonide 200 mg is as follows:
Excipients: Alcohol 2 ml, Saccarose 50 g, Glicerol 10g, Sodio saccarinate 80mg, Carbossimetilcellulosa sodica 40mg, Sodio fosfato bibasico 735 mg, Nipagina 100 mg, Nipasolo 20 mg, Aroma 0,2 mg, and water qb a 100 ml.
According to Darren Svirskis, et al. in their 2020 article, “Alcohol-free Extemporaneous Formulations of Furosemide Are Chemically and Physically Stable in Ora-Blend Products for 30 Days”, it states “Formulations containing 2 mg/mL furosemide were prepared by crushing furosemide tablets and mixing with commercial vehicles Ora-Blend, Ora-Blend SF, or SyrSpend-SF Alka” [14].
From Bayview Pharmacy, “Aspirin 20 mg/ml Oral Suspension is a liquid dosage form that contains aspirin uniformly dispersed throughout a liquid medium” [15].
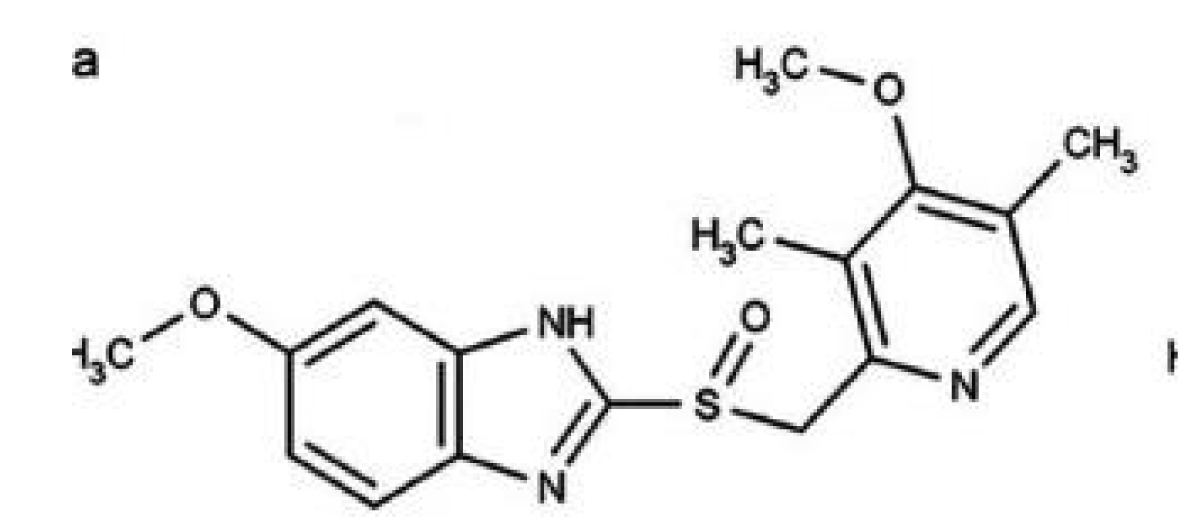
The UTIFAR Technical Italian Union of Pharmacists stated that the oral suspension niaprazine 3mg/ ml, niaprazina 3 mg/mL, and niaprazina 300 mg is comprised of BASE L. x SOSP. pH 4 ml 500 CILIEGIA qb a 100.0 ml (expiration date is 90 days if used this ready-for-use exipients formulation) (Figure 15).
Figure 15: NIAPRAZIN chemical formula.
From the Secundum artem “STABILITY OF EXTEMPORANEOUSLY PREPARED ORAL LIQUID FORMULATIONS”, it was mentioned that “The Ursodiol 25 mg/mL oral liquid was prepared by counting out ten 300 mg ursodiol capsules. The contents of the capsules were emptied into a glass mortar and comminuted well. Ten mL of glycerin was added and the mixture levigated to form a smooth paste. Sixty mL of Ora-Plus was added geometrically with mixing until a smooth mixture was obtained. The mixture was transferred to a calibrated 120 mL amber bottle. A small quantity of orange syrup was added to the mortar to rinse the remaining drug mixture and added to the bottle. Sufficient orange syrup was added to volume and mixed well.
From the results of this study, a beyond-use date of up to 60 days can be used for this preparation. The initial and final pH values for both temperatures did not vary outside the range of pH 3.5 to pH 3.7.13” [16].
From the SIFAP Italian Society of Preparatory Pharmacist galenic procedures [17], we can see the formulation of Amoxicillin Oral Suspension as shown in Figure 16.
Figure 16: Amoxicillin oral suspension formulation 250 mg/5 ml. (Formulation is in Italian) [17].
From UTIFAR, the example of one basis for suspension ready-for-use in commerce, and the advantages for galenic pharmacists are as follows:
- It simplifies the work of API slightly soluble in water;
- It makes it possible to prepare pharmaceutical forms safe, stable, and easy to be taken for patients with difficulty in swallowing.
- It makes it possible to personalize the therapy and dosing for a wider target of patients (pediatric-geriatric);
- It improves the rheologic property of the preparation helping to prevent the sedimentation of the API, and making the homogenization easier for all components to assure maximum accuracy of the dosage;
- They have a pleasant taste, good palatability, and high masking power.
- They are without gluten, lactose, and other allergens.
- They are without sugar and ethanol, heavy metals, or other solvents.
Recently, USP pharmacopeia prioritized a list of formulations for the development of CPMs for pediatric patients, like aspirin compounded oral suspension 230 mg/ml, Captopril, carvedilol, hydrocortisone, bosentan, nadolol, and others.
For the preparation of magistral oral suspension, mortar, and pestle are to be used to treat and triturate the solid powders API and the hydrocolloid. Then it is necessary to use the sift, then the wetting excipients must be added for mixing, and the preserved water is added till the final volume.
If tensioactives are present, they must be dissolved beforehand in a small amount of preserved water. If gum or cellulose derivates are used, the API is mixed in a mortar and worked with a pestle with this mucillagine adding a little portion of preserved water, with other water solution of the other component since the total final volume requested. In order to favor the preparation the polysaccharide can be wetted with hydrophilic liquids like glycerol, alcohol, and sorbitol (all excipients and their concentrations must be present like the formulation requested).
From the SIFAP procedure for IBUPROFEN oral susp 100mg/5ml, “the pharmacists can evaluate for the preparation of ibuprofen oral suspension the use of ready-for-use bases like: Base liquid as per suspension Acef; Base per suspension oral Galeno; Fast Oral Solution Wagner – Farmalabor, SyrSpend SF PH4 Fagron; or other similar”.
From an observational point of view, some relevant literature is reported as well as some formulations and commercial products like the ready-for-use bases. The reported Figures 1-18 helped in clarifying some concepts. An experimental project hypothesis is submitted and after all this, is a global conclusion reported related to the topic of this work.
From the literature of Sachin S. Gaikwad, et al. “Exploring paediatric oral suspension development: Challenges, requirements, and formulation advancements” (2024), “The main reason for the development of pharmaceutical suspensions PS is because of drug poor water solubility. Additionally, suspensions also aim to prevent the bitter taste of the API -therapeutic ingredients, and increase the oral absorption and bioavailability, as well as the drug stability” [18].
According to Oriana Boscolo, et al. (2020), “It is well known that the chemical stability of OMZ omeprazole is a function of pH. OMZ is rapidly degraded at pH value below 7.4, but it is stable at alkaline condition (pH 9.5).4 For this r, OMZ is commercialized as oral dosage form like enteric-coated tablets ECT or extended-release capsules containing enteric-coated granules or pellets. The enteric coating protects OMZ from acid degradation in the stomach” [19] (Figure 17).
Figure 17: Structure of Omeprazole.
A study by Paul A Whaley, et al. states that “Omeprazole OMZ is available in both tablet and capsule form, with varying strengths of each. The need for other administration options for those patients who cannot take tablets or capsules has led compounding pharmacies to seek other alternatives. One alternative is the use of a suspending agent to create an oral solution or suspension OS. In the past, this has been accomplished using a sodium bicarbonate solution as the vehicle. Sodium bicarbonate/omeprazole OMZ combination imparts a bitter and unpleasant taste. SyrSpend SF Alka (reconstituted) is a vehicle for making a suspension which has a pleasant taste, thus increasing palpability and compliance” [20].
According to Christine M Geiger, et al. (2015), “The API was considered stable if the suspension retained 90% to 110% of the initial concentration. Furosemide was stable for at least 14 days in the SyrSpend SF Alka at refrigerated conditions. Prednisolone sodium phosphate in the SyrSpend SF PH4 was stable for at least 30 days at room temperature and refrigerated conditions. Ranitidine hydrochloride suspensions in the SyrSpend SF PH4 at room temperature and refrigerated conditions were stable for at least 30 days and 58 days, respectively. Hydrocortisone hemisuccinate and sodium phosphate retained greater than 90% for at least 60 days at both room temperature and refrigerated samples in the SyrSpend SF PH4. Amiodarone hydrochloride and nifedipine suspensions at both room temperature and refrigerated conditions retained greater than 90% of the initial concentrations for at least 90 days in SyrSpend SF PH4. Refrigerated samples of simvastatin in SyrSpend SF PH4 were stable for at least 90 days. Spironolactone in the SyrSpend SF PH4 at room temperature retained more than 90% of the initial concentration for at least 90 days. Phenobarbital in the SyrSpend SF PH4 retained above 90% of initial concentration for at least 154 days at room temperature [21].
In 2023, Mercedeh Mansourian, et al. stated that “The stability of amoxicillin trihydrate oral suspension in SyrSpend® SF PH4 was investigated under storage conditions of 2 °C - 8 °C and 25 °C for 30 days. The results demonstrated that the formulation remained stable throughout the study duration in both the temperature conditions. At 25 °C, a decline of 5.62% was observed after 30 days, although no accompanying physical changes were noted” [22].
Marianne Bobillot, et al. mentioned that “in pediatric and neonatal units, oral liquid forms OLF are widely used, as solid forms are contraindicated before the age of 6 due to anatomical and neurological immaturity [23].
In a work by Khadija Rouaz, et al. it was specified that “The most toxic excipients in neonates are known to be Na benzoate, propylene glycol, methyl para hydroxybenzoate, propyl, sodium saccharine, benzyl alcohol, benzalkonium chloride, polysorbate 80 and ethanol. These excipients are used in formulations according to the study conducted” [24].
A study by Antonio Spennacchio, et al. showed that “to provide appropriate therapeutic care for pediatric patients, BU could be extemporaneously formulated as viscous oral suspensions VOS. The main limit of this therapy is the low residence time of the formulation on the esophageal mucosa that is not sufficient for the drug, which is suspended in a liquid vehicle, to solubilize and diffuse into the mucosa exerting its local anti-inflammatory action. This limitation is even more pronounced because of BU’s low water solubility. To overcome this kind of problem, a new ready-to-use mucoadhesive viscous liquid vehicle, named Fast Oral Solution Wagner (B1), has been used. Utilizing ready-to-use liquid vehicles is a valuable strategy for creating extemporaneous formulations” [25].
Experimental project
In a public hospital (PC area) of about 700 beds and with pediatric wards and neonatal pathology, the study’s time of observation was 6 months from January to June 2024. The study was on all pediatric requests for oral solution or suspension (classic formula vs ready-for-use bases).
The proportion of the amount of prepared Oral solution/suspension: 1/5
Total preparations = 100 (including captopril, propranolol, flecainide, furosemide, sulfadiazin, pirimetamin, niaprazin, and hydrocortisone).
Collection of data: It was carried out by the galenic pharmacist.
Evaluation method: Verified all written major non-conformity related prepared orals suspension or solution.
Results: There were no reports of written major non-conformity for both of these preparations in the same galenic lab.
One signal of incomplete dissolution for an oral solution with an API with a low water solubility (Sulfadiazin) and one relating a break of an oral suspension produced using ready-for-use bases in proximity with the expiration date (pirimetamin). It is to be noted that the collection of the written non-conformity is an admitted procedure in hospital settings.
It is clear that oral suspension in galenic practice is a really versatile kind of pharmaceutical form. It is easy to take for children, is available in various ready-for-use excipients, and is easily prepared in the galenic labs. It is clear also that capsule preparation is a more complex procedure and takes more time than oral suspension. As innovations are available like the ready-for-use bases that make it possible to produce in an easier way for this oral suspension, it is necessary to verify the compatibility of the basis with the API; gastrosensible or not; the chemical-physical global compatibility; and microbiological stability.
The same is necessary to verify the possibility of their use or not; the specific excipient and their concentration related to the age of the patients. In every way, it is mandatory on the label to write the concentration in mg/ml to avoid errors in subministration. To be added the label Narcotics if used related API. Also, it is needed to verify the dose-related age or body weight in the prescription to avoid dangerous overdosage (especially for API with strictly therapeutic indexes like Flecainide or others).
The final bottle must be in preference way of dark glass in order to avoid light inactivation of some API. The expiry date is 30 days, but these limits can be reduced or increased if specific measures are adopted for microbial protection and related to the stability of the oral suspension.
The producers of ready-for-use vehicles provide generally written information about this, for example, 14 days for captopril without ascorbic acid as an antioxidant, or 30 -60 -90 days for other API-related specific ready-for-use base.
The temperature of conservation can be at 2-8 grades for some API and room temperature for others based on global stability. In order to guarantee the right dosing it is needed to add the label on the bottle “NEEDED TO BE MIXED BEFORE THE USE” (Figure 18).
Figure 18: A “Shake well before use” label.
Special instructions: 1. Shake well before use (before taking the right dose with a graduated syringe); 2. Store at an adequate temperature kept out of reach of children. A specific safety closing system is needed to protect the children. Mandatory to label with the conservation modality (temperature, light). Needed to provide a syringe dispenser graduated in order to make it possible to measure the amount to be subministrated. The expiry data to verify the indication of the producer of the ready-for-use bases related to the specific API used or pharmacopeia prescription for this kind of liquid preparations. The normative rules (26-30) are to be followed as well as the excipient characteristics [31].
Because oral suspensions (OS) are a versatile kind of galenic preparation, the pediatry can ask for the preparation of oral suspension rather than cps when possible. This makes it possible to reduce the complexity of the galenic lab reducing time and costs. This is also because, in commerce, there are various ready-for-use bases for many APIs (also for gastro sensible). But it is mandatory to verify the compatibility of these bases with the ready-for-use product or to ask the pediatry. A classic oral solution formula with all excipients written into the prescription. The related local practical experience submitted presents no difference in the report of major non-conformity between the classic formula and ready-for-use bases.
In conclusion, the pharmacist can use ready-for-use bases for oral suspension verifying the compatibility and safety with the specific API in order to simplify the procedure for OS.
- Pharma Central. Pharmaceutical suspending agents: Overview, types, and selection criteria. 2021. Available from: https://pharmacentral.com/learning-hub/technical-guides/pharmaceutical-suspending-agents/
- Sheskey PJ, Hancock BC, Moss GP, Goldfarb DJ, editors. Handbook of pharmaceutical excipients. 9th ed. London: Pharmaceutical Press; 2020. Available from: https://www.amazon.in/HANDBOOK-PHARMACEUTICAL-EXCIPIENTS-9ED-2020/dp/0857113755
- Morg S. Enhancing pediatric care: The importance of oral suspensions in pediatric drug delivery. Short Communication. 2024;8(1):1-4. Available from: https://www.primescholars.com/articles/enhancing-pediatric-care-the-importance-of-oral-suspensions-in-pediatric-drug-delivery-128106.html
- Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Dan Med Bull. 1999 Jun;46(3):183–96. PMID: 10421978. Available from: https://pubmed.ncbi.nlm.nih.gov/10421978/
- Allegany Nutrition. The human digestive tract pH range diagram. Available from: https://www.alleganynutrition.com/supporting-pages/the-human-digestive-tract-ph-range-diagram/
- Patel VP, Desai TR, Chavda BG, Katira RM. Extemporaneous dosage form for oral liquids. Pharmacophore. 2011;2(2):49-66. Available from: https://pharmacophorejournal.com/storage/models/article/NTH2w2N4ScGJbvhjMITiyuTZwUHwBCnXNqVtWElOnODmDwLffAfAaeabhyFL/extemporaneous-dosage-form-for-oral-liquids.pdf
- Nationwide Children’s Hospital. Pharmacy services. Captopril compound sheet. 2020. Available from: https://www.nationwidechildrens.org/specialties/pharmacy-services/compounding-formulas
- US Pharmacist. Captopril 1 mg/ml oral solution. Available from: https://www.uspharmacist.com/article/captopril-1-mg-ml-oral-solution
- Grissinger M. Life-threatening errors with flecainide suspension in children. Pharm Ther. 2018 May;43(5):258. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5912238/
- Sickkids Pharmacy. Propranolol 5 mg/ml oral suspension sheet. Available from: https://www.sickkids.ca/siteassets/care--services/for-health-care-providers/compounding-recipes/propranolol-5mgml-pharmacy-compounding-recipe.pdf
- Silva MR, Dysars LP, Santos EP, Ricci E. Preparation of extemporaneous oral liquid in the hospital pharmacy. Braz J Pharm Sci. 2020 Apr 6;56. Available from: http://dx.doi.org/10.1590/s2175-97902019000418358
- US Pharmacist. Sildenafil citrate 25 mg/ml oral liquid. 2018. Available from: https://www.uspharmacist.com/article/sildenafil-citrate-25-mg-ml-oral-liquid
- Pharmaceutical Society of New Zealand. New Zealand standardised oral formulation batch sheet. Hydrocortisone suspension 1 mg/mL (100 mL). Available from: https://www.psnz.org.nz/Folder?Action=View%20File&Folder_id=189&File=NZSOF%20Hydrocortisone%201mg-1mL.pdf
- Svirskis D, Jaffer J, Agarwal P, Khan A, Kaur J, Cheng A, Hanning S. Alcohol-free extemporaneous formulations of furosemide are chemically and physically stable in Ora-Blend products for 30 days. Int J Pharm Compd. 2020 ;24(3):246-251. Available from: https://pubmed.ncbi.nlm.nih.gov/32401745/
- Bayview Pharmacy. Aspirin 20 mg/ml oral suspension. Available from: https://www.bayviewrx.com/formulas/Aspirin-20-mg-ml-Oral-Suspension-Heart-Disease-Stroke-Prevention-Rheumatoid-Arthritis-Osteoarthritis-Kawasaki-Disease
- Secundum Artem. Stability of extemporaneously prepared oral liquid formulations – Part V. Curr Pract Compounding Inform Pharm. 2010;14(3):1-10. Available from: https://www.padagis.com/wp-content/uploads/2021/10/Sec-Artem-14.3.pdf
- SIFAP - Società Italiana Farmacisti Preparatori. Istruzione operativa per l’allestimento di amoxicillina sospensione orale. Available from: https://www.sifoweb.it/images/pdf/attivita/attivita-scientifica/aree_scientifiche/Area_galenica/I.O._Amoxicillina_Sosp.Orale_Rev.01_del_22.05.pdf
- Gaikwad SS, Morales JO, Lande NB, Catalán-Figueroa J, Laddha UD, Kshirsagar SJ. Exploring pediatric oral suspension development: Challenges, requirements, and formulation advancements. Int J Pharm. 2024;657:124169. Available from: https://doi.org/10.1016/j.ijpharm.2024.124169
- Boscolo O, Perra F, Salvo L, Buontempo F, Lucangioli S. Formulation and stability study of omeprazole oral liquid suspension for pediatric patients. Hosp Pharm. 2020 Oct;55(5):314-322. Published online 2019. Available from: https://doi.org/10.1177%2F0018578719844704
- Whaley PA, Voudrie MA, Sorenson B. Stability of omeprazole in SyrSpend SF (reconstituted). Int J Pharm Compd. 2012;16(2):164-166. Available from: https://pubmed.ncbi.nlm.nih.gov/23050328/
- Geiger CM, Sorenson B, Whaley P. Stability assessment of 10 active pharmaceutical ingredients compounded in SyrSpend SF. Int J Pharm Compd. 2015;19(5):420-427. Available from: https://pubmed.ncbi.nlm.nih.gov/26775449/
- Mansourian M, Dijkers E, Silva CCV, Vale HC. Compatibility of commonly used active pharmaceutical ingredients in a ready-to-use oral suspending vehicle. Pharmaceutics. 2023;15(10):2388. Available from: https://doi.org/10.3390%2Fpharmaceutics15102388
- Bobillot M, Delannoy V, Trouillard A, Kinowski JM, Sanchez-Ballester NM, Soulairol I. Potentially harmful excipients: State of the art for oral liquid forms used in neonatology and pediatrics units. Pharmaceutics. 2024;16(1):119. Available from: https://doi.org/10.3390/pharmaceutics16010119
- Rouaz K, Chiclana-Rodríguez B, Nardi-Ricart A, Suñé-Pou M, Mercadé-Frutos D, Suñé-Negre JM, et al. Excipients in the pediatric population: A review. Pharmaceutics. 2021;13(3):387. Available from: https://doi.org/10.3390/pharmaceutics13030387
- Spennacchio A, Lopalco A, Racaniello GF, Cutrignelli A, la Forgia FM, Fontana S, et al. Mucoadhesive budesonide solution for the treatment of pediatric eosinophilic esophagitis. Pharmaceuticals. 2024;17(5):550. Available from: https://doi.org/10.3390/ph17050550
- Italian National Tariffario. Available from: https://www.aifa.gov.it/en/web/guest/tariffe
- European Pharmacopoeia. Available from: https://www.edqm.eu/en/european-pharmacopoeia
- US Pharmacopoeia. Available from: https://www.usp.org/
- British Pharmacopoeia. Available from: https://www.pharmacopoeia.com/
- Italian Farmacopea Ufficiale. 12th ed. Rome: 2008. Available from: https://search.worldcat.org/title/849324852
- ILM DELLA SALUTE. Italian DM Salute 18.11.2003. Available from: https://www.aifa.gov.it/sites/default/files/DM_Salute_18.11.2003.pdf