More Information
Submitted: August 28, 2024 | Approved: September 04, 2024 | Published: September 05, 2024
How to cite this article: de Souza BVC, Ribeiro AB, Rita de Cássia MO, Portela JVF, Ana Amélia de Carvalho MC, Barros EML, et al. Nanoencapsulated Extracts from Leaves of Bauhinia forficata Link: In vitro Antioxidant, Toxicogenetic, and Hypoglycemic Activity Effects in Streptozotocin-induced Diabetic Mice. Arch Pharm Pharma Sci. 2024; 8(1): 100-115. Available from:
https://dx.doi.org/10.29328/journal.apps.1001063
DOI: 10.29328/journal.apps.1001063
Copyright License: © 2024 de Souza BVC, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction inany medium, provided the original work is properly cited.
Keywords: Bauhinia forficata Link; Bioactive compounds; Antioxidant capacity; Antidiabetic activity
Nanoencapsulated Extracts from Leaves of Bauhinia forficata Link: In vitro Antioxidant, Toxicogenetic, and Hypoglycemic Activity Effects in Streptozotocin-induced Diabetic Mice
Bárbara Verônica Cardoso de Souza1, Alessandra Braga Ribeiro2*, Rita de Cássia Meneses Oliveira3, Julianne Viana Freire Portela4, Ana Amélia de Carvalho Melo Cavalcante5, Esmeralda Maria Lustosa Barros3, Luís Felipe Lima Matos6, Tarsia Giabardo Alves7, Maria do Carmo de Carvalho e Martins3 and Lívio César Cunha Nunes6
1Nutrition Department, Federal University of Piaui, Teresina, Piaui, Brazil
2Portuguese Catholic University, CBQF Center for Biotechnology and Fine Chemistry–Associated Laboratory, School of Biotechnology, Portugal
3Medicinal Plants Research Center, Federal University of Piaui, Teresina, Piaui, Brazil
4Nutrition Department, Federal University of Piaui, Picos, Piaui, Brazil
5Laboratory of Genetical Toxicology, Federal University of Piaui, Teresina, Piaui, Brazil
6Center for Pharmaceutical Technology, Federal University of Piaui, Teresina, Piaui, Brazil
7Histology and Embryology Department, Federal University of Piaui, Teresina, Piaui, Brazil
*Address for Correspondence: Alessandra Braga Ribeiro, Portuguese Catholic University, CBQF Center for Biotechnology and Fine Chemistry–Associated Laboratory, School of Biotechnology, Rua Diogo Botelho 1327, 4169-005 Porto, Portugal, Email: [email protected]
In this study, we evaluated the assessed the hypoglycemiant, toxicogenetic and genotoxic effects of nanoencapsulate extracts of Bauhinia forficata Link. Phytochemical evaluations of extracts were carried out, as well as the evaluation using HPLC-MS and of antioxidant capacity in vitro. DM2 was induced in mice with streptozotocin and extracts were given orally for 28 days. That dried extract from infusion (ESIN) had a higher rate of bioactive compounds compared to the dried extract from decoction (ESDC), and higher antioxidant capacity. Glucose levels decreased from 77.26% to 57.79% and 45.15% after supplementation with ESIN (200 and 600 mg/kg/day) and ESDC (600 mg/kg/day), respectively, when compared to the diabetic group treated with metformin (600 mg/kg/day) (21.53%), with an improvement in the glycemic response e recovery of pancreatic β cells. Thus, our study has shown that these extracts exhibit hypoglycemiant activity, with a beneficial effect superior to metformin, as a result they could be considered as potential therapeutic agents for application in pharmaceutical formulations in the treatment of DM2.
Type 2 diabetes mellitus (T2DM) is a highly prevalent disease that affects more than 500 million people worldwide and is estimated to reach 1 billion people by 2050, with diabetes type 2 being responsible for over 90% of all cases. Despite the current knowledge of its risk factors and many public policies with prevention strategies, its incidence keeps increasing globally, which is why research on DM2 and drug development is encouraged in the search for novel alternatives to improve the quality of life for these patients [1,2].
Hyperglycemia is the main characteristic of DM2, caused by a deficiency in insulin due to a dysfunction in pancreatic β cells and resistance to insulin on tissues, with high morbimortality and rise of long-term complications, including retinopathy, nephropathy, damage to peripheral nerves, and cardiovascular diseases, leading to functional impairment and depression on patients, besides the financial burden on the family due to insulin costs, glycemic monitoring, oral antidiabetic medications and other drugs, constituting a relevant economic impact on countries and health systems [3].
Regarding DM2 pathogenesis, concomitant to hyperglycemia and hyperlipidemia, oxidative stress has played a key role, considering that in DM, the mitochondria are the main generators of reactive oxygen species (ROS) [4,5]. The increase in the production of ROS induces alterations in the expression and activity of antioxidant enzymes, making tissues more susceptible to oxidative stress, which leads to the development of diabetic complications [6]. Thus, antioxidants such as flavonoids, which eliminate free radicals, can help prevent or fight against DM2 and its aggravations, since they act on the metabolism of glucose, decreasing glycemia [7-9].
Bauhinia forficata Link is a medicinal plant, belonging to the family Fabaceae, largely used in forms (infusion and decoction) as a hypoglycemia in popular medicine [10]. Several studies reported the pharmacological effects of its leaves associated with the presence of flavonoids, mainly quercetin, and kaempferol. Antidiabetic effects as well as the prevention of aggravations associated to oxidative stress were attributed to a derivative of kaempferitrin [10-22].
Kaempferitrin also induces cytotoxic, antitumor, antiproliferative, and chemopreventive effects in vitro and in vivo against cells derived from human tumors of cervical carcinomas (HeLa), through apoptosis via the intrinsic caspase-dependent pathway and induces cell cycle arrest in the G1 phase [23]. Cechinel-Zanchett et al [24], showed that pre-treatment with the flavonoid kaempferitrin decreased the cytotoxicity of irinotecan in intestinal epithelial cells (IEC-6) and intestinal damage in mice exposed to irinotecan, due to its antioxidant potential, with consequent chemo preventive effect.
The cytotoxic effects of B. forficata extract were also evaluated in non-cancer cells by in vivo and in vitro systems, Artemia salina and human lymphocytes, respectively, showing no toxicity. However, in a human melanoma cell line (FO-1), B. forficata extracts significantly reduced the growth of these cancer cells, inferring a potential selectivity of this medicinal plant in inhibiting the growth of cancer cells [25].
There are some commercially available drugs for the treatment of DM. However, some of them are not accessible to the population, in addition to demonstrating adverse effects on health such as gastrointestinal disorders, hypoglycemia, weight gain, cardiovascular diseases, and urinary tract infections. Thus, B. forficata Link can be a viable alternative as a coadjutant in the treatment of DM2, with minimal side effects, and relatively low cost compared to synthetic medicines [26,27]. Its use as nanoencapsulated dried extracts to be applied in pharmaceutical forms is very promising when compared to fluids, due to lower storage costs and higher concentration and stability of the bioactive substances [28].
However, most studies with this plant species were conducted using fresh leaves or extracts obtained by organic solvents [29,30]. Thus, the present work used dry nanoencapsulated extracts, obtained from aqueous extracts of the leaves of B. forficata, in which the antioxidant activity, as well as the antidiabetic activity, in mice, in addition to its toxicogenetics through hematological, biochemical, and histopathological parameters and its genotoxic effects in multi-organs for application in novel pharmaceutical formulations, as a coadjutant in the treatment of DM2.
Plant material, extract preparation, chemical products and reagents
The leaves of Bauhinia forficata Link were collected during the year 2018, in Piaui, Brazil (5°03’7.2” S 42°77’26.4” W). A voucher specimen for leaves was deposited at Herbarium Graziela Barroso of the University Federal do Piaui, with registration at SISGEN AE1E536, as suggested by Chan, et al. [31]. Leaves were submitted to drying in an industrial stove at a temperature of 40 ºC, for 52 h, and were then pulverized in a mill. Aqueous extracts were obtained through decoction and infusion, in a proportion of 10% (m/v), according to Menezes, et al. [32]. The drying process of the extracts was carried out using a BUCHI B-290 spray-dryer, under the following conditions: inlet temperature 110 °C, flow rate 5.4 mL/min, 15% injection, pressure 0.9 bar and spray at 70%. The concentration of encapsulating agents was 30% (15% colloidal silicon dioxide and 15% maltodextrin), obtained through a factorial design 22, according to Rolim Neto, et al. [33], added to extractive solutions, consisting of 70% of plant material. After nanoencapsulation, the nanocapsulated extracts were named extract infusion extract (ESIN) and decoction dry extract (ESDC) and were previously characterized in a previous study by De Souza, et al. [34], regarding the physical-chemical, spectroscopic parameters, in addition to the bioaccessibility of its phenolic compounds, through in vitro digestion and its antioxidant activity, to identify differences between these extracts. All the chemical products and reagents required for this study were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA), and HPLC reagents were purchased from Merck KGaA (Darmstadt, Germany).
Phenolic compounds and antioxidant capacity
Total polyphenols were analyzed according to the method proposed by Swain and Hills [35]. Total flavonoids were quantified by the aluminum trichloride (AlCl3) method, according to Zhishen, Mengcheng, and Jianming [36]. Antioxidant capacity was measured through the DPPH· (2,2-diphenyl-1-picrylhydrazyl) radical-scavenging assay, the 2,2’-azinobis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS•+) radical cation assay, ferric reducing antioxidant power (FRAP) and by the oxygen radical absorption capacity (ORAC) method. The (DPPH) radical scavenging assay was performed according to Kim, et al. [37]. Antioxidant activity, through the ABTS free radical scavenging, was conducted according to Re, et al. [38]. Ferric reducing antioxidant power (FRAP) was assessed using the method proposed by Arnous, et al. [39]. ORAC assay was conducted according to the described methods of Prior, Hoang, Gu [40] and Dávalos, Gómez-Cordovés, Bartolomé [41].
Analysis by high-performance liquid chromatography coupled to mass spectrometry - HPLC-MSn
Main total-polyphenol and flavonoids were separated, identified, and quantified by high-performance liquid chromatography through a photodiode array detector coupled to electrospray ionization sequential mass spectrometry and ion trap analyzer (HPLC-PDA-ESI-IT-MSn), according to Cuyckens and Claeys [42]. Samples were filtered and 20 μL were injected into the HPLC system, in triplicates. The mobile phase consisted of water, 0.1% formic acid (A), and acetonitrile (B) and ran at a flow rate of 0.5 mL.min-1. Polyphenols and flavonoids were identified using strategies alongside an analysis of the mass spectra obtained by the HPLC-PDA-ESI-IT-MSn, comparison to a database of mass spectrum like the Massbank® (https://massbank.eu/MassBank/) and literature data [43,44].
Animals
A total of 55 male mice (Mus musculus), Swiss strain, weighing between 20 to 30 grams, were obtained from the Central Bioterio of the Federal University of Piaui. Animals were kept in a controlled environment for temperature (23° ± 1°C), in a 12-hour light/dark cycle with free access to water (ad libitum) and to commercial food for rodents. The experiment protocol was approved by the Ethics Committee on Animal Experimentation of UFPI (CEEA/UFPI N° 008/12) and developed following the guidelines of well-being and biosafety in animal experimentation proposed by the Brazilian Society of Science in Laboratory Animals and the National Council for Control of Animal Experimentation.
Toxicogenetic study
Acute toxicity: Acute oral toxicity studies were conducted in agreement with the Guidelines of the Organization for Economic Cooperation and Development (OECD) [45]. Mice were divided into four groups (n = 5), in which two of them received an aqueous solution containing nanoencapsulated dried extracts of the leaves of B. forficata Link subsp. forficata (ESIN2 and ESDC2); another group received a solution solely with the drying adjuvants (ADJV2); and a negative control group (NA), received only distilled water. The administered dose, via orogastric gavage, was 2000 mg/kg of the body weight.
Animals were monitored for the analysis of signs of toxicity through Hippocratic screening, which provides a general estimative for the toxicity of the substance under a state of conscience and general disposition, activity and motor system coordination, reflexes, and activities controlled by the central and autonomic nervous systems according to Malone and Robichaud [46] and Cunha, et al. [47].
This monitoring took place during the first hour after administration of the substances and periodically, in the first 24 h, with special attention during the first 4 h, the experiment was conducted in three moments: the first day (T0), seventh day (T7), and fourteenth day (T14), also, weight assessment was carried out in these three instances. The parameters assessed were the screening of the general activity of the animal, vocal tremor, irritability, force feedback, tail grip, twisting, body tone, strength in grasping, negative coordination, corneal reflex, tremors, convulsions, hypnosis, ptosis (inclination or fall upper eyelid), urination, defecation, piloerection (involuntary erection or bristling of the hair), breathing and mortality as Brito [48] and Silva, et al. [49]. Animals were placed in metabolic cages to monitor feed intake, water, and excreta elimination for 14 days.
Genotoxic assessment: For genotoxic assessment, during the acute toxicity study, a comet assay was carried out in different tissues and peripheral blood. The alkaline version of the comet assay was performed according to Dos Santos, et al. (2012) and Speit, and Rothfuss [50]. 10 μL aliquots of caudal peripheral blood of male mice were collected on the seventh (T7) and the fourteenth (T14) days of treatment and submitted to the comet assay. On the 14th day of treatment, 10 μL aliquots of cell homogenate of bone marrow, pancreas, liver, and peripheral blood were also collected and submitted to the comet assay.
Cell material was mixed with a thin layer of 0.75% low melting point agarose (90 μL) and smeared on pre-coated slides with 1.5% negative control agarose. The slides were then dipped in lysis solution (2.5 M NaCl, 100 mM EDTA, and 10 mM Tris, pH 10 with the addition of 1% Triton X-100 and 10% DMSO at the time of use) for up to 72 hours at 4 ºC. After this period, slides were incubated in an alkaline buffer (300 mM NaOH and 1 mM EDTA, pH > 13) for 20 minutes; then, they were exposed to an electric current of 300 mA and 25 V (0.90 V/cm) for 15 minutes in an electrophoresis chamber.
Finally, slides were neutralized with Tris buffer (0.4 M and pH 7.5) and stained with silver nitrate solution. Results were expressed as damage index (DI) and frequency of damage (%FD). DI was calculated using the formula: DI = Σ (number of cells in a given damage class X damage class), which ranged from 0 to 400. FD was calculated using the following formula: FD = 100 - number of class 0 cells. Bone marrow was collected from the epiphysis with fetal bovine serum, and centrifuged at 1500 rpm. After collection from the liver and pancreas, the organs were macerated in PBS, centrifuged, and subjected to the aforementioned procedure.
Hematological parameters: After the period of the experiment, animals were anesthetized using sodium thiopental (40 mg.kg-1, intraperitoneally (i.p.)) and received lidocaine pre-analgesia (10 or mg.kg-1, i.p.). Blood samples were collected by cardiac puncture and conditioned in microtubes with EDTA anticoagulant (tetra acetic acid) at the concentration of 10 mg/dL to analyze the following hematological parameters: erythrocytes, hematocrit, hemoglobin, total leukocyte count, neutrophils, eosinophils, basophils, monocytes and platelets, using the Advia 120/hematology (Siemens) automated hematology analyzer according to Ferreira, et al. [51].
Data for the toxicogenetic study were analyzed using the statistical software Graphpad prism (version 6.0).
Antidiabetic activity
Induction of diabetes: A total of 35 male mice were submitted to diabetes induction through intraperitoneal injection of a freshly made solution of streptozotocin (STZ, Sigma Chemical Company, Saint Louis, MO, USA) [52], at a single dose at a concentration of 150 mg/kg of the weight, dissolved in 0.01 M citrate buffer (pH 4.5), after a 12-hour fasting, according to Salgueiro, et al. [18]. Healthy mice received the same volume of the vehicle (citrate buffer). Seventy-two hours after induction, the glucose levels in the blood of the mice were measured by collecting a drop of blood from the tip of the tail and submitting it to an On-Call Plus glucometer. Mice with fasting blood glucose levels≥ 240 mg/dL were considered diabetic for this experiment.
Experiment design
After the DM2 model was established, mice were divided randomly into seven groups with five animals each, according to Matteucci and Giampietro [53]. The negative control group (NA) and the diabetic group (DA) were only administered distilled water; another diabetic group (DM) was treated using metformin, at a dose of 600 mg/kg/day, and treatment groups received ESIN and ESDC at doses of 200 mg/kg/day (ESIN2 and ESDC2), and 600 mg/kg/day (ESIN6 and ESDC6).
The doses used in this study were determined from the result of the toxicity tests, and also guided by the doses used in previous studies conducted by Da Cunha, et al. [13], and Pereira, et al. [54]. All samples were administered orally through an orogastric cannula, for 28 days, and animals were weighed weekly. Blood samples from the caudal vein were collected every seven days, after a night fasting of eight hours, to estimate blood sugar levels.
Oral Glucose Tolerance Test (OGTT): During the last week of treatment, mice were submitted to OGTT after a night fast of 12 hours, according to Pan, et al. [55] and Zhu, et al. [9]. We administered an oral solution of glucose (aqueous solution at 40%) at a dose of 2 mg/kg body weight. Then, blood was collected from the caudal vein of the mice at times 0, 30, 60, 90, and 120 minutes after administration of glucose, which was conferred using the glucometer.
Biochemical parameter: Biochemical tests were performed with blood samples, following Ribeiro, et al. [56], in which blood was centrifuged at 3000×g for 10 min at 4 ºC, immediately, and serum was obtained to determine levels of blood glucose, total cholesterol (TC), triglycerides (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea and creatinine. Assays were conducted using an automatic machine Labmax 240 with commercial systems from LABTEST®. After the death of the animal, the heart, lungs, stomach, liver, pancreas, and kidneys were collected, then cleaned with 0.9% NaCl solution and weighed to calculate the relative index of each organ. The last three organs were fixed with 10% formaldehyde for histopathological analysis.
Histopathological analysis: Proceedings followed by Li, et al. [2] [57] and Wang, et al. [58], in which tissue from the liver, kidneys, and pancreas was dehydrated in crescent series of ethanol (70 – 100%), diaphanized in xylol, followed by inclusion in histological paraffin. The inclusion blocks were sectioned in a standard microtome at a thickness of 3 μm and the sections obtained were stained using hematoxylin and eosin; the stained slides were examined under an electron microscope.
Statistical analysis
Results were presented as the mean ± standard deviation or standard error of the mean (S.E.M.). Data were analyzed by the t-student test for phenol content and antioxidant capacity, analysis of variance (ANOVA), Tukey test for the remaining experiments, and Bonferroni test for genotoxic assessment. These data, as well as correlations and graphs, were analyzed utilizing the program GraphPad Prism (version 6.0), with a 95% confidence level (p < 0.05).
Phenolic compounds and antioxidant capacity
Table 1 displays the quantification of polyphenols, flavonoids, and the antioxidant capacity of nanoencapsulation dried extracts ESIN and ESDC, through the DPPH·, ABTS•+, FRAP, and by the method ORAC. Total polyphenols and flavonoids content showed a significant statistical difference between extracts, making it possible to infer that ESIN (29.98 mg EAG.g-1 and 9.78 EC. g-1) contains more bioactive compared to ESDC (21.49 mg EAG.g-1 and 3.06 EC. g-1). Results from this study exhibited superior flavonoid values to the ones obtained by Da Cunha, et al. (2010) for dried extracts of B. forficata leaves in an oven and by spray drying.
| Table 1: Quantification of polyphenols, flavonoids and the antioxidant capacity of dried extracts from of B. forficata Link. | ||
| ESIN | ESDC | |
| Polyphenols (mg EAG. g-1)1 | 29,98 ± 0,08a | 21,49 ± 0,14b |
| Flavonoids (9,78 EC. g-1)2 | 9,78 ± 0,28a | 3,06 ± 0,03b |
| DPPH· (μmol Trolox.g-1 )3 | 101,60 ± 0,35a | 64,72 ± 0,47b |
| ABTS•+ (μmol Trolox.g-1)3 | 8,12 ± 0,01a | 7,11 ± 0,01b |
| FRAP (μmol Trolox.g-1)3 | 118,86 ± 0,82a | 57,68 ± 0,93b |
| ORAC (μmol Trolox.mg-1)4 | 1465,63 ± 1,97a | 1102,03 ± 0,92b |
| Values are presented as means ± standard deviation. ESIN: Dry Infusion Extract; ESDC: Dry Extract of the Decoction. a, b different letters on the same line show statistically significant differences between the extracts (p < 0.05; t-student test); 1 expressed in mg gallic acid equivalent.g-1 of the total polyphenols (PT) of dry extracts; 2 mg of catechin equivalent.g-1 of the total flavonoid (FT) extracts; 3 μmol trolox. g-1equivalent of the dried extracts; 4 μmol trolox equivalent. mg-1 of sample. | ||
Concerning the antioxidant activity of these extracts, we also observed statistically significant differences between them in all assays (DPPH, ABTS, FRAP and ORAC), in which ESIN displayed more activity (101.60 μmol Trolox.g-1, 8.12 μmol Trolox.g-1, 118.86 μmol Trolox.g-1, 1465.63 μmol Trolox.mg-1) when compared to ESDC (64.72 μmol Trolox.g-1, 7.11 μmol Trolox.g-1, 57.68 μmol Trolox.g-1, 1102.03 μmol Trolox.mg-1), showing that the types of flavonoids extracted during the infusion process had a better response to oxidative stress (Table 1).
In our study, both showed antioxidant capacity in the ORAC assay which was superior to ascorbic acid (1136.0 μmol trolox eq/g) and to other ethanolic extracts obtained from the same plant species (855.8 μmol trolox eq/g) in a study by de Franco, et al. [59], implying that the process of extraction and nanoencapsulation of the studied extracts preserved the content of phenolic compounds and their antioxidant activity. These results are relevant, as this assay provides some benefits when compared to other antioxidant methods, such as the fact that it is conducted in physiological temperature and pH and the use of fluorescence instead of absorbance reduces the interference of colored compounds present in the samples, as reported by Dávalos, Gómez-Cordovés, Bartolomé [41] and Prior, Hoang, Gu [40].
ESIN and ESDC extracts can be a potential source of antioxidant compounds that play a role in detoxing the body, eliminating reactive oxygen or nitrogen species caused by hyperglycemia, a characteristic of DM, suggesting that this antioxidant capacity contributes positively to the prevention and treatment of this pathology, besides acting as signaling molecules in the mitochondrial endoplasmic reticulum and enzymatic pathways of metabolism. Therefore, it is relevant to assess the content of phenolic compounds as well as the antioxidant activity of plant extracts to verify their antidiabetic activity [60,61].
Analysis by HPLC-PDA-ESI-IT-MSn
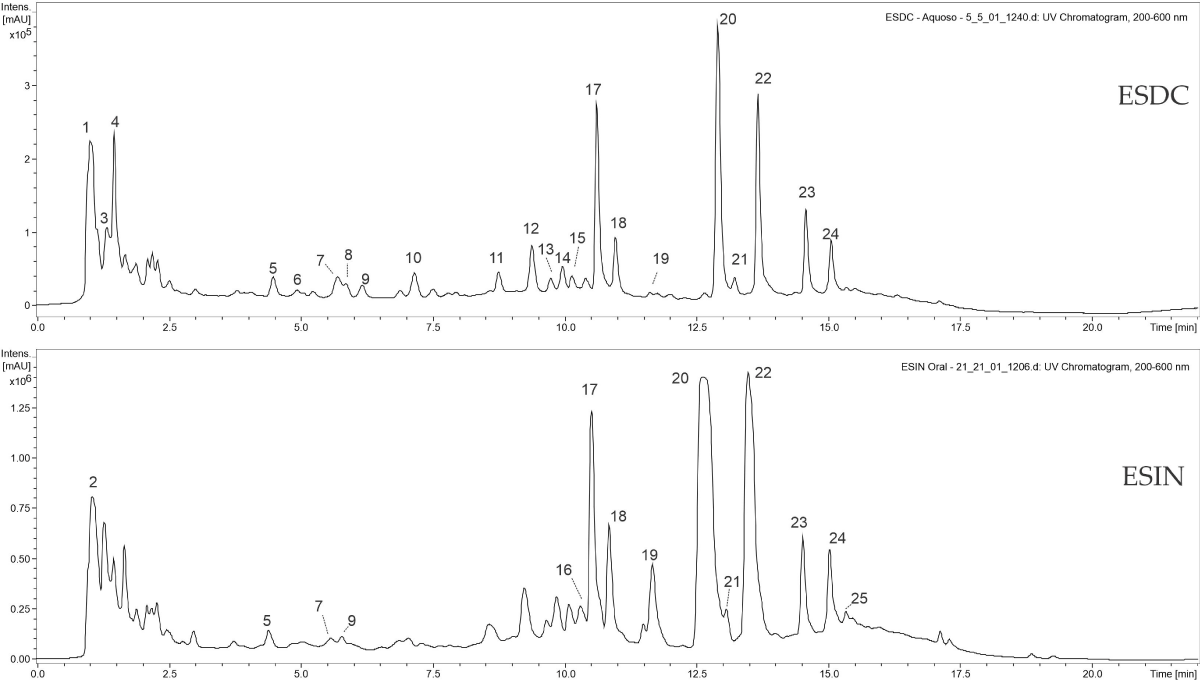
During the chromatographic analysis, phenolic acids and their glycosylated derivatives (between 8-10 minutes) and C- and O-glycosylated flavonoids within 10-20 minutes of the chromatogram (Figure 1) were mainly identified in the studied extracts. Da Cunha, et al. [13], Da Silva e Filho [62], Filho [63], and Pizzolatti, et al. [16], in their studies with extracts of B. forficata leaves, also found that phenolic compounds, especially flavonoids, are the main constituents.
Figure 1: Chromatograms of flavonoids and their O-glycosylated derivatives present in the ESDC and ESIN of the leaves of Bauhinia forficata Link [34].
Peaks number 11, 20, and 23 to 28 showed UV spectra with absorption bands between 254 and 365 nm, characteristic of flavonoids. Peak number 11 presented m/z 447 as its non-protonated ion, and its second-order fragmentation (MS2[447]) gave rise to ion m/z 315 as a product, due to the loss of an O-glycosylated pentose ([M ̶ 132 ̶ H]-), where m/z 315 refers to the aglycone of flavonol isorhamnetin via the break on the Y0 bind, a compound also identified by Farag, et al. [42] in their study with eight Bauhinia species.
Peaks 23 and 24 may be two isomers of kaempferol-O-rhamnosyl-3-O-rutinoside due to sequential fragments similar to peak 20 ((MS2[739]: m/z 593 and (MS3[739-593]: m/z 285), which are shown to be isomeric molecules with each other, having kaempferol such as aglycone and sequential losses of rhamnose and a rutinose. Farias e Mendez [64], Ferreres, et al. [43], Santos, Fortunato, and Spotorno [65], have also identified flavonoid derivatives in extracts of this same plant species, including kaempferol glycosides, whose ions have been identified as flavonoids-O-glycosides.
Toxicogenetic
Acute toxicity: For acute toxicity, a recommended maximum dose of 2000 mg/kg of the body weight was, then mice were observed regarding general behavioral changes, toxicity symptoms, and mortality after treatment during the first 4 hours, which are the most critical; then, during 24 hours and, posteriorly, for 14 days. At the end of the experiment, we did not register any deaths or observable evidence of behavioral toxicity.
Pereira, et al. [53] also conducted an acute toxicity study with a hydroalcoholic extract of B. forficata Link, at this same dose and did not record any deaths, indicating that this extract does not show relative toxicity at the test concentration. Düsman, et al. [66], verified that this plant holds a good potential to be used as a safe medicament, since they showed that its aqueous extract did not exhibit cytotoxic activity on bone marrow cells of Wistar rats, besides contributing to reducing chromosomic damage induced by chemotherapy due to its antimutagenic activity on pre-treatment (71%), simultaneous treatment (91%) and post-treatment (95%) with cyclophosphamide. Pepato, et al. [67], in their study with diabetic and regular rats that received aqueous decoction of B. forficata leaves at the concentration of 150 g/L, orally, for 33 days, also did not observe any measurable toxic effects with the enzymatic markers used.
Systemic toxicity, besides the Hippocratic screening, can be verified through the decrease in consumption of water and food, body weight, and relative weight of organs, according to Teo, et al. [68]. Body weight is one of the most commonly used parameters for toxicological assessments to indicate the often early appearance of toxic effects of a given substance in animal organisms. Lu, et al. [69] considered the gain or loss of body mass of toxicological importance if this alteration is of at least 10%. Acute administration of ESDC2 and ESIN2 did not reduce any of the assessed parameters in comparison with negative control (NA) (Table 2), indicating no significant toxicity of these extracts.
| Table 2: Means of body weight, feed consumption, water and excreta in mice treated with acute dose of nanoencapsulated dried extracts of Bauhinia forficata Link in mice. | ||||
| Parameters | NA | ADJV2 | ESDC2 | ESIN2 |
| Body weight (g) | 25,13 ± 0,25 | 26,49 ± 0,64 | 28,73 ± 0,17* | 28,24 ± 0,69* |
| Feed consumption(g) | 10,00 ± 0,86 | 9,23 ± 0,65 | 8,76 ± 1,34 | 9,88 ± 0,31 |
| Water consumption (mL) | 22,87 ± 2,23 | 26,00 ± 1,14 | 30,50 ± 1,32* | 31,10 ± 1,05* |
| Excrete (g) | 4,58 ± 0,15 | 2,92 ± 0,50 | 3,56 ± 0,62 | 4,03 ± 0,73 |
| Values represent means ± S.E.M. NA: Negative Control; ADJV: Animals treated with adjuvants at the dose of 2000 mg / kg. ESDC2: Animals treated with dry extract of the decoction at the dose of 2000 mg / kg. ESIN2: Animals treated with dry extract of the infusion at the dose of 2000 mg / kg. Differences in the experimental groups were determined by ANOVA, one-way, followed by Tukey post-test. * p < 0.05 compared to the NA group. | ||||
Genotoxic: Results from the genotoxic monitoring conducted during acute toxicity can be seen in Tables 3 and 4.
| Table 3: Genotoxic effects and repair of nanoencapsulated dried extracts of Bauhinia forficata Link evaluated by theindex and frequency of damage in peripheral blood of mice. | ||
| Groups | Oral administration (2000 mg/kg) | |
| Damage index (0 a 400) | Frequency of damage (%) | |
| NA | 50,00 ± 4,50 | 32,80 ± 7,19 |
| CPA | 317,00 ± 51,92**a | 99,80 ± 0,44***a |
| ADJV2 (T7) | 317,00 ± 20,17***a | 99,40 ± 0,49***a |
| ESIN2 (T7) | 232,20 ± 12,26***a | 93,00 ± 3,14**a |
| ESDC2 (T7) | 237,60 ± 14,91***a | 93,60 ± 2,96**a |
| ADJV2 (T14) | 153,00 ± 37,06**bc | 61,80 ± 9,44*b*c |
| ESIN2 (T14) | 124,20 ± 5,97*bc | 56,00 ± 8,61**b*c |
| ESDC2(T14) | 151,60 ± 30,04*bc | 49,40 ± 9,86**b*c |
| Values representmeans ± standard deviations (100 cells perexperiment). NA: Negative Control; CPA: Cyclophosphamide(50 mg / kg) (positive control).ADJV: Animals treated with adjuvants at the dose of 2000 mg / kg. ESIN2:Animals treated with dry extract of the infusion at the dose of 2000 mg / kg;ESDC2: Animals treated with dry extract of the decoction at the dose of 2000mg/kg. T7: Seventh day after administration. T14: 14th day afteradministration. Differences in the experimental groups were determined byANOVA, two-way, followed by Bonferroni post-test. * p < 0.05, ** p < 0.01,*** p < 0.001. a in relation to the NA group, b inrelation to the CPA group, c in relation to the T7. | ||
| Table 4: Genotoxic effects of nanoencapsulated dried extracts of Bauhinia forficata Link evaluated by the index and frequency of damage in the bone marrow, liver and pancreas of mice, after 14 days of treatment. | ||||||
| Groups | Damage index (0-400) | Frequency of damage (%) | ||||
| Bone marrow | Liver | Pancreas | Bone marrow | Liver | Pancreas | |
| NA | 52,20 ± 5,40 | 54,80 ± 5,26 | 52,40 ± 5,03 | 35,00 ± 12,02 | 45,80 ± 6,38 | 33,60 ± 6,87 |
| CPA | 273,20 ± 57,22***a | 264,40 ± 44,61***a | 264,40 ± 55,92***a | 94,20 ± 5,80**a | 89,00 ± 7,17**a | 87,20 ± 5,45***a |
| ADJV2 | 161,20 ± 7,43***a | 103,40 ± 7,12*a | 63,20 ± 9,52 | 88,60 ± 5,77*a | 77,00 ± 5,56*a | 44,00 ± 4,18 |
| ESIN2 | 150,60 ± 2,88***a | 112,20 ± 12,40**a | 54,20 ± 9,52 | 72,40 ± 2,07*a | 81,60 ± 3,57*a | 54,40 ± 6,06 |
| ESDC2 | 166,80 ± 16,10***a | 110,40 ± 12,93**a | 61,20 ± 12,07 | 82,00 ± 3,93*a | 77,16 ± 6,04*a | 51,80 ± 3,49 |
| Values represent means ± standard deviations (100 cells per experiment). NA: Negative control; CPA: Cyclophosphamide (50 mg / kg) (positive control); ADJV: Animals treated with adjuvants at the dose of 2000 mg / kg; ESIN2: Animals treated with dry extract of the infusion at the dose of 2000 mg / kg; ESDC2: Animals treated with dry extract of the decoction at the dose of 2000 mg / kg; Differences in the experimental groups were determined by ANOVA, two-way, followed by Bonferroni post-test. * p < 0.05, ** p < 0.01, *** p < 0.001. a in relation to the NA group. | ||||||
Index and frequency of DNA damage in the peripheral blood of animals, after seven days of extract administration, demonstrated significant differences concerning negative control (NA); however, we observed that genotoxic DNA damages were repaired after 14 days of administration when compared with the positive control (CPA).
In the bone marrow of mice treated with the extracts, genotoxic effects were observed, at the dose of 2000 mg/kg, in groups ADJV2, ESIN2, and ESDC2, with damage indexes of 161 ± 7.43, 150.60 ± 2.88, and 166 ± 16.10, respectively. We also observed damage in the liver, yet they were inferior to those caused by cyclophosphamide (CPA) (264 ± 44.61) when compared to negative control (NA) (54.80 ± 5.26). However, these effects were not observed in the pancreas of animals of groups treated with extracts ESIN2 (54.20 ± 9.52) and ESDC (61.20 ± 12.07), with no statistical difference concerning negative control (NA) (54.40 ± 5.03).
De Sousa, et al. [70] and Varela‐Barca, Agnez‐Lima, and De Medeiros [71], in their studies with Bauhinia species, have shown divergent results about the mutagenic potential of its extracts, which can be explained by the different chemical compounds that might be present, depending on the type of extract. Hodek, Trefil, Stiborová [72] and Skibola and Smith [73] found that flavonoids may exhibit antioxidant or mutagenic activity depending on the number and the position of the hydroxyl groups on rings A and B, as well as the types of solvents used for the extraction of these compounds.
Effects of ESDC and ESIN on the relative weight of organs: Relative weight of the main organs of mice, treated with a single dose of ESIN and ESDC, did not show significant alterations when compared to negative control (NA). During the 28 days of treatment, we observed a reduction of the pancreas in the following group’s DM, ESIN2, and ESDC6 when compared to NA, due to diabetes. There was no alteration in the liver amongst treated animals, with similar sizes to NA, except for ESIN6 which had an increase, which was also observed for the lungs and the stomach (Table 5).
| Table 5: Relative weights of organs (g/100 of body weight) of mice treated with nanoencapsulated dried extracts of Bauhinia forficata Link in mice. | ||||||||
| Organs | Oral administration (14 dias) (2000 mg/kg) | |||||||
| NA | ADJV2 | ESIN2 | ESDC2 | |||||
| Liver | 6,14 ± 0,26 | 3,61 ± 0,85 | 4,26 ± 0,30 | 5,60 ± 0,40 | ||||
| Pancreas | 0,91 ± 0,13 | 0,45 ± 0,09 | 0,30 ± 0,06 | 1,54 ± 0,68 | ||||
| Kidneys | 1,37 ± 0,19 | 1,20 ± 0,26 | 1,14 ± 0,23 | 1,53 ± 0,07 | ||||
| Heart | 0,70 ± 0,02 | 0,46 ± 0,09 | 0,37 ± 0,08 | 0,50 ± 0,01 | ||||
| Lungs | 0,78 ± 0,01 | 0,54 ± 0,11 | 0,54 ± 0,11 | 0,99 ± 0,30 | ||||
| Stomach | 1,08 ± 0,06 | 0,69 ± 0,15 | 0,69 ± 0,14 | 0,81 ± 0,08 | ||||
| Organs | Oral administration (28 dias) (200 mg/kg e 600 mg/kg) | |||||||
| NA | DA | DM | DSIN2 | DSIN6 | DSDC2 | DSDC6 | ||
| Liver | 5,31 ± 0,18 | 6,12 ± 1,04 | 4,66 ± 0,38 | 5,20 ± 0,55 | 8,50 ± 0,56* | 5,19 ± 0,34 | 5,21 ± 0,26 | |
| Pancreas | 0,84 ± 0,08 | 0,49 ± 0,08 | 0,42 ± 0,05* | 0,28 ± 0,10* | 0,65 ± 0,14 | 0,61 ± 0,06 | 0,47 ± 0,06* | |
| Kidneys | 1,12 ± 0,15 | 1,31 ± 0,29 | 0,94 ± 0,24 | 0,75 ± 0,19 | 1,86 ± 0,18 | 0,90 ± 0,17 | 0,94 ± 0,17 | |
| Heart | 0,58 ± 0,04 | 0,57 ± 0,09 | 0,39 ± 0,05 | 0,45 ± 0,05 | 0,88 ± 0,15 | 0,35 ± 0,01 | 0,49 ± 0,06 | |
| Lungs | 0,70 ± 0,02 | 0,71 ± 0,07 | 0,51 ± 0,05 | 0,69 ± 0,07 | 1,22 ± 0,11* | 0,59 ± 0,03 | 0,84 ± 0,05 | |
| Stomach | 0,92 ± 0,03 | 0,97 ± 0,18 | 0,83 ± 0,10 | 0,76 ± 0,28 | 1,77 ± 0,23* | 0,97 ± 0,06 | 0,95 ± 0,04 | |
| Relative weights calculated on the basis of the absolute weight of the organ (g) by the live weight value (g) x100. Values represent means ± S.E.M. NA: Negative control; ADJV: Animals treated with adjuvants at the dose of 2000 mg / kg. ESDC2: Animals treated with dry decoction extract at the dose of 2000 mg / kg; ESIN2: Animals treated with dry infusion extract at the dose of 2000 mg/kg. Differences in the experimental groups were determined by ANOVA, one-way, followed by Tukey post-test. * p < 0.05 compared to the NA group. | ||||||||
Effects of ESDC and ESIN on the hematological parameters: Hematological parameters were analyzed, as they are important criteria to assess the extension of homeostasis and alterations resulting from pathological and toxicity processes, as reported by Okonkwo, et al. [74]. We observed a decrease in neutrophils and an increase in lymphocytes of all groups treated with the maximum dose of 2000 mg/kg/weight when compared to NA. This is justified by the fact that bioactive compounds, at high doses, go from antioxidants to pro-oxidants, which can cause inflammation and induce apoptosis, although this latter effect can be beneficial when applied in the treatment of neoplasms, according to Cerqueira, De Medeiros and Augusto [75].
During the 28-day treatment, leucocytes increased in groups DM, ESIN6, and ESDC2, an alteration that can be attributed to diabetes, which is a chronic inflammatory disease, as reported by Asmat, Abad, and Ismail [6]. Lymphocytes and neutrophils also showed alterations on DN, showing that this group, treated with metformin (600 mg/kg/weight), was the one that displayed more alterations (Table 6).
| Table 6: Hematological parameters after oral administration of nanoencapsulated dried extracts of Bauhinia forficata Link in mice. | ||||||||
| Parameters | Oral administration (14 dias) (2000 mg/kg) | |||||||
| NA | ADJV2 | ESIN2 | ESDC2 | |||||
| HEM (106/uL) | 9,3 ± 0,5 | 7,8 ± 0,3 | 6,9 ± 0,6 | 8,7 ± 0,7 | ||||
| HGB (g/dL) | 14,5 ± 0,4 | 14,9 ± 0,5 | 14,5 ± 0,4 | 16,2 ± 0,6 | ||||
| HTC (%) | 53,5 ± 1,6 | 45,8 ± 5,2 | 42,5 ± 4,7 | 49,6 ± 0,5 | ||||
| LTC (103/uL) | 2522,0 ± 163,9 | 3115,0 ± 425,0 | 2913,3 ± 403,5 | 2235,0 ± 8 | ||||
| NTL (%) | 55,3 ± 9,3 | 3,6 ± 2,1* | 9,6 ± 1,2* | 5,5 ± 0,3* | ||||
| LTL (%) | 31,0 ± 5,5 | 78,2 ± 14,1* | 86,4 ± 3,2* | 74,2 ± 10,0* | ||||
| MNT (%) | 12,0 ± 2.0 | 5,2 ± 4,1 | 2,8 ± 0,1 | 12,2 ± 0,2 | ||||
| ESL (%) | 0,7 ± 0,3 | 0,1 ± 0,0 | 0,1 ± 0,0 | 0,7 ± 0,1 | ||||
| BSL (%) | 3,7 ± 0,6 | 12,8 ± 7,8 | 4,3 ± 0,5 | 12,4 ± 4,1 | ||||
| PLT (103/uL) | 803,0 ± 25,0 | 789,0 ± 55,0 | 644,7 ± 42,0 | 592,0 ± 116,0 | ||||
| Parameters | Oral administration (28 dias) (200 mg/kg e 600 mg/kg) | |||||||
| NA | DA | DM | DSIN2 | DSIN6 | DSDC2 | DSDC6 | ||
| HEM (106/uL) | 9,5 ± 0,2 | 9,6 ± 0,6 | 8,7 ± 0,4 | 7,9 ± 0,6 | 8,9 ± 0,3 | 9,2 ± 0,3 | 9,4 ± 0,2 | |
| HGB (g/dL) | 14,6 ± 0,3 | 14,5 ± 0,8 | 14,5 ± 0,5 | 13,5 ± 0,8 | 13,7 ± 0,5 | 15,7 ± 0,6 | 14,70 ± 0,3 | |
| HTC (%) | 52,7 ± 0,8 | 52,6 ± 4,3 | 52,2 ± 0,9 | 46,3 ± 4,9 | 44,7 ± 3,2 | 51,3 ± 1,8 | 51,87 ± 1,3 | |
| LTC (103/uL) | 2290,5 ± 40,1 | 2210,0 ± 92,9 | 3265,7 ± 198,2* | 2310,3 ± 59,9 | 3810,0 ± 109,7* | 3321,7 ± 143,6* | 2578,0 ± 168,0 | |
| NTL (%) | 42,2 ± 11,3 | 36,4 ± 7,3 | 2,6 ± 0,7* | 28,7 ± 0,3 | 12,0 ± 1,4* | 2,8 ± 0,81* | 5,9 ± 1,4* | |
| LTL (%) | 46,7 ± 11,0 | 58,6 ± 8,2 | 88,4 ± 2,2* | 66,0 ± 8,7 | 81,0 ± 3,3 | 75,3 ± 9,82 | 77,6 ± 6,4 | |
| MNT (%) | 7,8 ± 1,9 | 4,0 ± 1,1 | 4,2 ± 1,3 | 4,5 ± 1,5 | 6,7 ± 1,8 | 3,9 ± 0,96 | 4,6 ± 1,4 | |
| ESL (%) | 0,3 ± 0,2 | 0,6 ± 0,2 | 0,6 ± 0,2 | 0,2 ± 0,25 | 0,0 ± 0,0 | 0,2 ± 0,02 | 0,15 ± 0,0 | |
| BSL (%) | 2,0 ± 0,6 | 0,4 ± 0,2 | 5,6 ± 0,9 | 0,5 ± 0,3 | 0,5 ± 0,2 | 7,9 ± 1,59* | 2,9 ± 1,0 | |
| PLT (103/uL) | 772,8 ± 28,9 | 1007,4 ± 80,9 | 832,5 ± 134,5 | 695,2 ± 73,8 | 835,0 ± 49,8 | 913,8 ± 49,4 | 826,1 ± 46,9 | |
| HEM: Red Blood Cells; HGB: Hemoglobin; HTC: Hematocrit; LTC: Leukocytes; NTL: Neutrophils; LTL: Lymphocytes; MNT: Monocytes; ESL: Eosinophils; BSL: Basophils and PLT: Platelets. Values represent means ± S.E.M. Differences in the experimental groups were determined by ANOVA, one-way, followed by Tukey post-test. *p < 0.05 compared to the NA group. | ||||||||
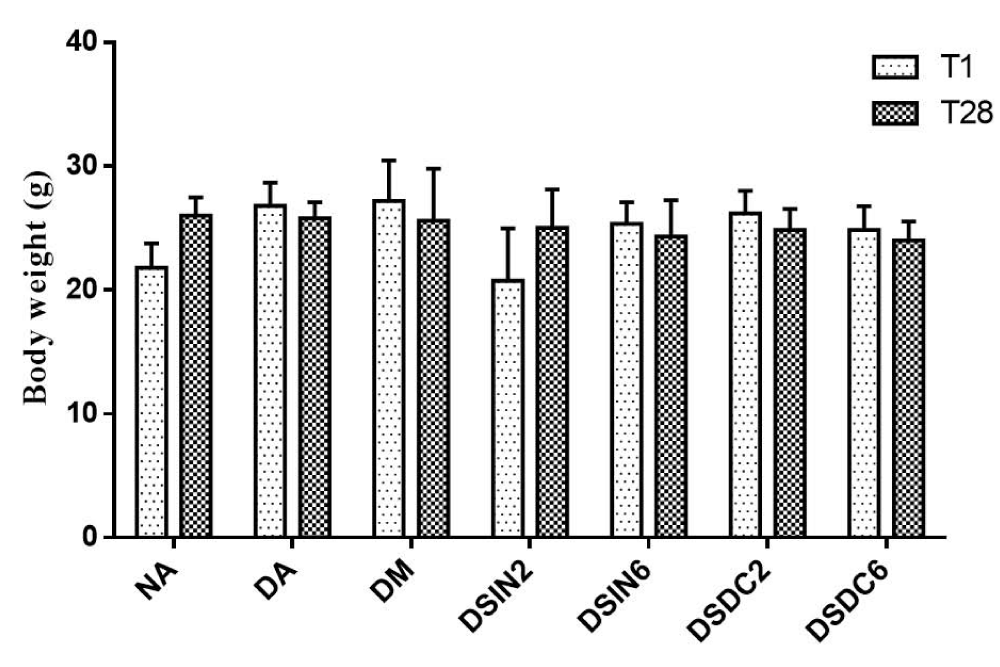
Effects of ESDC and ESIN on weight, blood glucose levels, and oral glucose tolerance: Figure 2 shows the effect of extracts ESIN and ESDC on body weight, which did not differ statistically between treated groups concerning the negative control group (NA), both on day 1 and day 28. A study conducted by Martínezmartínez, et al. [76] with diabetic mice showed that these animals also did not exhibit a significant weight gain during treatment with decoction from B. forficata leaves.
Figure 2: Effect of ESIN and ESDC on the body weight of diabetic mice. Values represent means ± S.E.M. NA: Negative control; DA: Untreated diabetic animals; DM: Diabetic animals treated with metformin at the dose of 600 mg/kg; DSIN2: Diabetic animals treated with dry infusion extract at the dose of 200 mg/kg; DSIN6: Diabetic animals treated with dry infusion extract at the dose of 600 mg/kg; DSDC2: Diabetics treated with dry decoction extract at a dose of 200 mg/kg; DSDC6: Diabetics treated with dry decoction extract at the dose of 600 mg/kg. Differences in experimental groups were determined by ANOVA, one-way, followed by Tukey post-test (p < 0.05). There was no statistically significant difference between the groups.
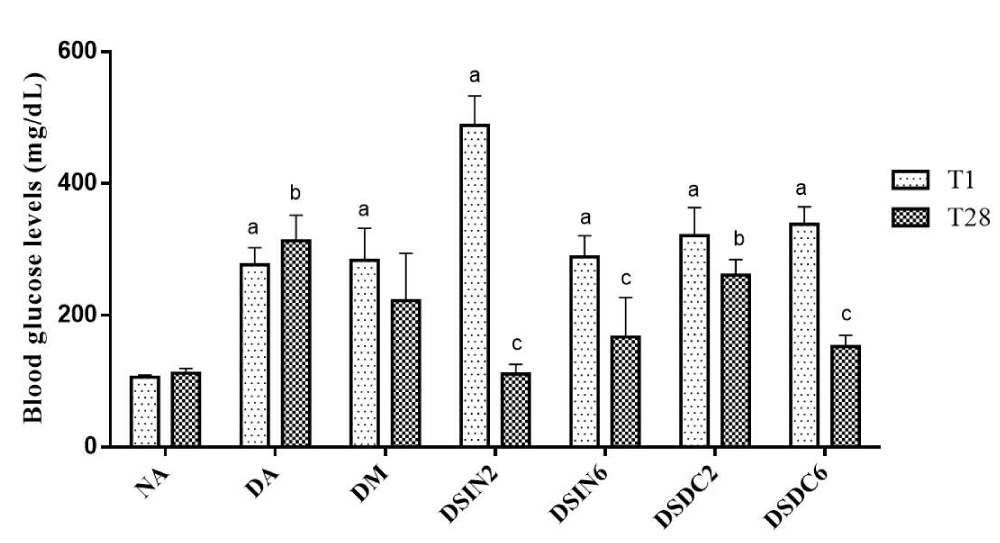
Fasting blood glucose levels of all experimental groups are shown in Figure 3. Blood glucose levels in the blood of diabetic groups showed a significant elevation in comparison to normal mice (NA) (p < 0.05), indicating the successful establishment of the DM2 model. After 4 weeks of supplementation with extracts ESIN and ESDC, blood glucose levels in groups ESIN2 (200 mg/kg/day), ESIN6 (600 mg/kg/day), ESDC6 (600 mg/kg/day) reduced significantly by 77.26%, 57.79% and 45.15%, respectively, when compared to the diabetic group treated with metformin (600 mg/kg/day), which only reduced its glycemia by 21.53% at the end of 28 days and did not demonstrate any statistical difference concerning group DA.
Figure 3: Effect of ESIN and ESDC in blood glucose from diabetic mice. Values represent means ± S.E.M. NA: Negative control; DA: Untreated diabetic animals; DM: Diabetic animals treated with metformin at the dose of 600 mg/kg; DSIN2: Diabetics treated with dry extract of the infusion at the dose of 200 mg/kg; DSIN6: Diabetic animals treated with dry infusion extract at the dose of 600 mg/kg; DSDC2: Diabetic animals treated with dry decoction extract at a dose of 200 mg/kg; DSDC6: Diabetic animals treated with dry decoction extract at the dose of 600 mg/kg; Differences in the experimental groups were determined by ANOVA, one-way, followed by Tukey post-test. p < 0.05 compared to NA in T1, bp < 0.05 compared to NA in T28, cp < 0.05 compared to DA in T28.
Amongst the groups treated with extracts, only group DSDC2 (200 mg/kg/day) did not obtain a reduction in blood sugar levels, being similar to the group with no treatment (DA). These results show the efficacy of nanoencapsulated extracts of B. forficata leaves in reducing blood sugar levels, in diabetic mice, at the two tested doses of the tested extracts ESIN and ESDC, except for the dose of 200 mg/kg/day of the latter, with both extracts showing blood glucose levels similar to those of non-diabetic group (NA) (p > 0.05), confirming that they are efficient in the treatment of DM2.
These results are relevant, as these extracts can be used by diabetic patients associated with a synthetic drug or to the use of insulin, to improve their efficacy and lead to treatment of DM2 using lower doses of synthetic drugs and hormones, as a result reducing side-effects, such as those caused by metformin for example (nausea, diarrhea, flatulence, inappetence and stomach pain), according to Cobble e Peters [77] and De Souza, et al. [27]. Additionally, extracts of some Bauhinia species can even improve gastrointestinal symptoms, mainly diarrhea, as reported in the study by Ahmed, et al. [78].
The beneficial effects of ESIN and ESDC extracts in the treatment of DM2 may be related to the various flavonoid glycosides, quercetin and kaempferol found in B. forficata leaves, as they act at multiple sites of carbohydrate metabolism in glucose regulatory pathways, improving insulin sensitivity, due to phosphorylation of the insulin receptor substrate (IRS), increasing insulin release, glucose uptake and glycogen biosynthesis, in addition to increasing adiponectin secretion, as already stated by Cazarolli, et al. [79], Cazarolli, et al. [80], Ren, et al. [81], Santos, Fortunato, Spotorno [65] and Sharma, Balomajumder, Roy [82]. As quercetin was not identified in the chromatographic analysis in our study, we can infer that the reduction in glycemia was due to kaempferol.
The hypoglycemic effect of these nanoencapsulated extracts can also be explained by the antioxidant action of the phenolic compounds released from the nanocapsules that act by removing or inactivating the peroxyl radicals (ROO•), formed during the initiation or propagation of the oxidation reaction, through the donation of atoms of hydrogen to these molecules, interrupting the chain reaction, inhibiting oxidative stress and its consequences [34].
The flavonoids present in these nanoencapsulation extracts (ESIN and ESDC), also modulate the gene expression of cytokines and enzymes such as α-amylase, α-glucosidase, lipase [58], in addition, to activate PPAR-γ, which promotes adipogenesis, as it induces the synthesis and storage of fatty acids and also inhibits the expression of inflammatory genes since iNOS suppresses the transcription factors AP-1 and NF-kB, modulates the MAPK activity and influences glucose uptake [83-86].
Alkhalidy, et al. [11] observed that a single daily dose of kaempferol, orally, can significantly improve hyperglycemia and increase glucose tolerance in insulin-deficient mice, which is associated with the increase in elimination and uptake of glucose, particularly oxidation in the muscular tissue and suppression of hepatic glucose production.
A study conducted by Da Cunha, et al. [13] with extracts of the leaves of B. forficata Link, dried by spray drying and oven drying and obtained using extraction solvents ethanol and water (1:2) as solvents, observed similar results to our present study, when finding a reduction in fasting glucose levels, after 7 days of treatment, at the dose of 200 mg/kg/body weight in STZ-induced diabetic mice.
A study on the antidiabetic activity in eight Bauhinia species was conducted by Farag, et al. [42] using the alpha-glucosidase inhibition assay, the results showed that the distinct species studied hold a potential antidiabetic effect, as they slow down the digestion of carbohydrates, resulting in lower post-prandial glucose levels and thus, being able to help in avoiding high peaks of glucose levels on DM2 patients. Ferreres, et al. [43] also confirmed the use of Bauhinia species as antidiabetic agents.
On the other hand, Volpato, et al. [87] orally administered, in pregnant and non-pregnant mice, an aqueous extract of B. forficata leaves, at doses of 500 mg/kg, 600 mg/kg and 1000 mg/kg, for 21 days, and found that this extract was not toxic at the tested doses but did not modify maternal hyperglycemia. Salgueiro, et al. [18], in their research with diabetic mice, treated diabetic mice daily for 21 days with the infusion from leaves of B. forficata subs. pruirosa, at the concentration of 313 mg/kg/weight and also did not observe a reduction in the blood glucose level in these animals, suggesting that kaempferitrin, the main substance responsible for hypoglycemic action, may have been lost during the preparation process of the infusion or is absent in this Bauhinia subspecies. These controversial results can also be justified by the differences in the experimental model used, types and methods of extract and/or fraction preparation, doses, and administration time, besides the genetic constitution of the species, environmental factors, and seasonal variations [76].
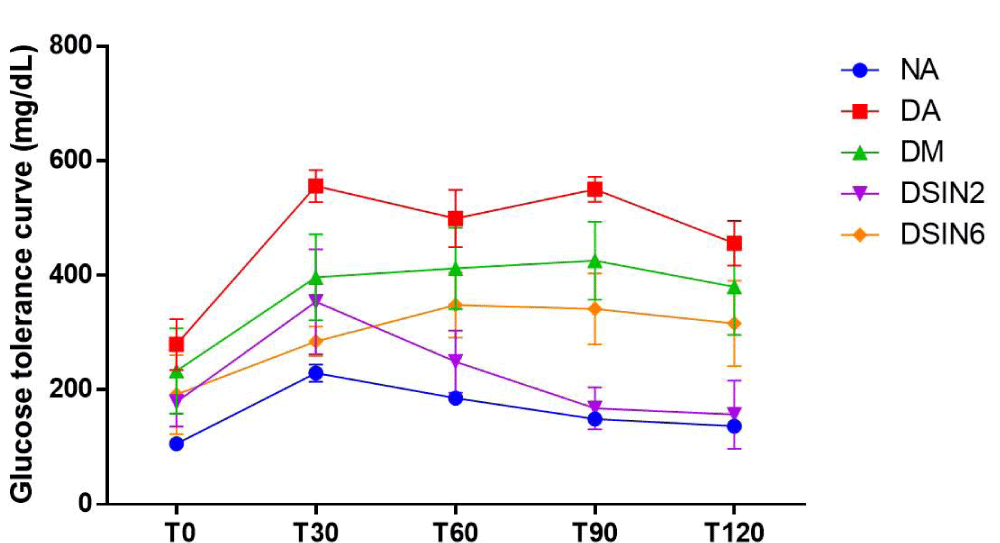
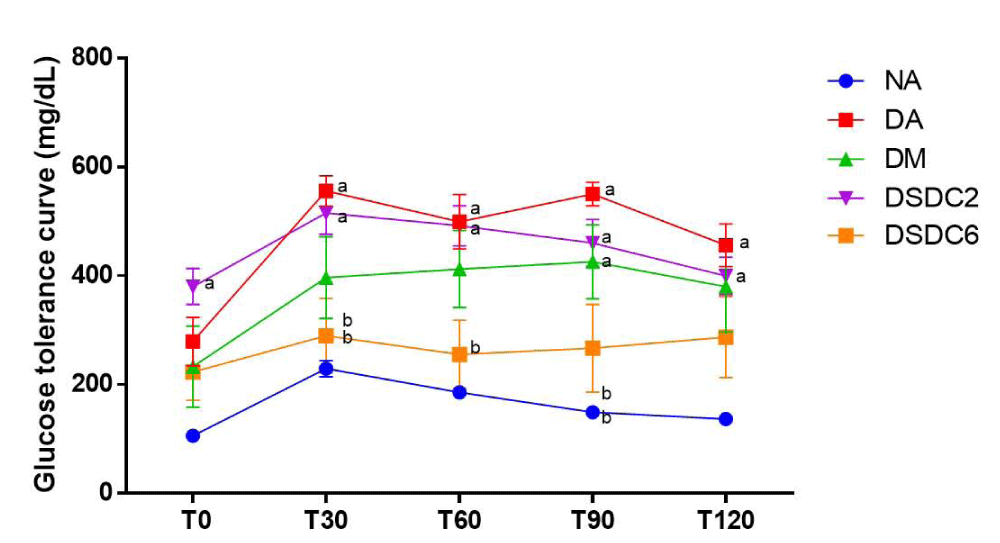
Effects of ESIN and ESDC on glucose tolerance in STZ-induced diabetic mice were shown in Figures 4 and 5. Oral glucose supplementation resulted in a gradual increase in blood glucose level within 30 min for all groups, remaining at an elevated level for the next 90 min, with animals from DSIN2 (168 mg/dL) already showing a decrease in blood glucose levels which is statistically different from the diabetic group without treatment (DA) (550 mg/dL) and with blood glucose levels values similar to group NA (149.33 mg/dL), that was permanent until the end of the experiment (T120), while glucose levels were slightly reduced for both (ESIN2 = 157.0 mg/dL and NA = 136.5 mg/dL), suggesting that glucose was being metabolized by insulin, reducing blood glucose levels in these groups.
Figure 4: Effect of ESIN on glucose tolerance of diabetic mice. Values represent means ± S.E.M. NA: Negative control; DA: Untreated diabetic animals. DM: Diabetic animals treated with metformin at the dose of 600 mg/kg. DSIN2: Diabetic animals treated with dry infusion extract at the dose of 200 mg/kg. DSIN6: Diabetic animals treated with dry infusion extract at the dose of 600 mg/kg; DSDC2: Diabetic animals treated with dry decoction extract at a dose of 200 mg/kg; DSDC6: Diabetic animals treated with dry decoction extract at the dose of 600 mg/kg; Differences in experimental groups were determined by ANOVA, one-way, followed by Tukey post-test (p < 0.05). p < 0.05 compared to NA, bp < 0.05 compared to DA.
Figure 5: Effect of ESDC on glucose tolerance of diabetic mice. Values represent means ± S.E.M. Values represent means ± S.E.M. NA: Negative control; DA: Untreated diabetic animals; DM: Diabetic animals treated with metformin at the dose of 600 mg/kg; DSIN2: Diabetic animals treated with dry infusion extract at the dose of 200 mg/kg; DSIN6: Diabetic animals treated with dry infusion extract at the dose of 600 mg/kg; DSDC2: Diabetic animals treated with dry decoction extract at a dose of 200 mg/kg; DSDC6: Diabetic animals treated with dry decoction extract at the dose of 600 mg/kg; Differences in experimental groups were determined by ANOVA, one-way, followed by Tukey post-test (p < 0.05). p < 0.05 compared to NA p < 0.05 compared to DA.
Concerning animal groups treated with ESDC, ESDC6 displayed a better glycemic response with blood glucose levels of 287.3 mg/dL, when compared to DSDC2 (399.5 mg/dL). It was confirmed in T28, with fasting blood glucose levels, that there was a reduction in blood glucose levels of animals treated with ESIN and ESDC, at the doses of 200 and 600 mg/kg/day except ESDC2.
Effects of ESDC and ESIN on lipid parameters, hepatic enzymes, and kidney function
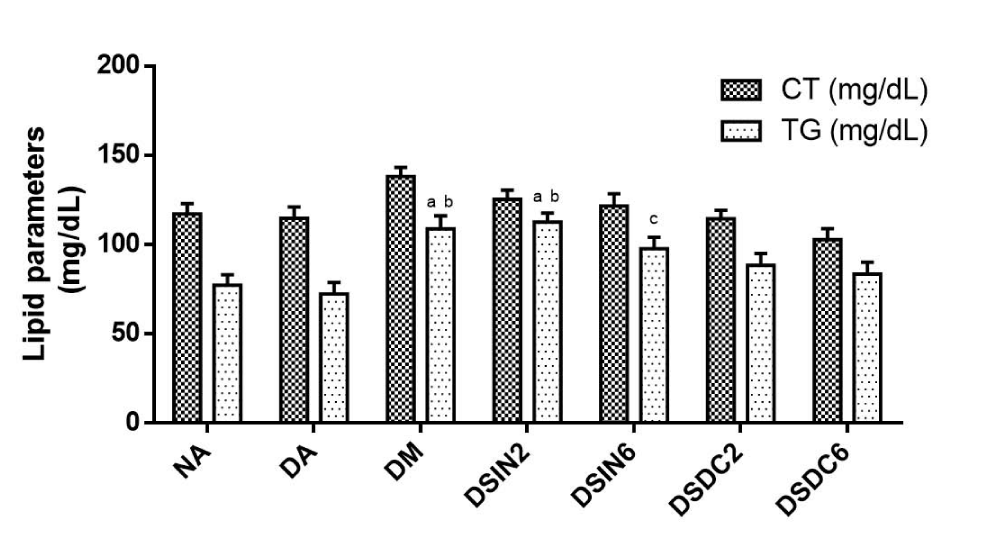
Total cholesterol (TC) levels for all groups were statistically similar concerning NA (117 mg/dL ± 5.95). Triglyceride levels (TG) of groups DA (72.40 mg/dL ± 6.33), DSIN6 (97.67 mg/dL ± 6.48), DSDC2 (88.40 mg/dL ± 6.75), and DSDC6 (83.40 mg/dL ± 6.62) showed levels similar to those of NA, with no observed significant statistical differences between the treated groups, only DM and DSIN2 displayed elevated levels compared to NA (Figure 6).
Figure 6: Effect of ESIN and ESDC on lipid parameters of diabetic mice. Values represent means ± S.E.M. Values represent means ± S.E.M. NA: Negative control; DA: Untreated diabetics; DM: Diabetic animals treated with metformin at the dose of 600 mg/kg; DSIN2: Diabetics treated with dry infusion extract at the dose of 200 mg/kg; DSIN6: Diabetic animals treated with dry infusion extract at the dose of 600 mg/kg. DSDC2: Diabetic animals treated with dry decoction extract at a dose of 200 mg/kg; DSDC6: Diabetics treated with dry decoction extract at the dose of 600 mg/kg; Differences in experimental groups were determined by ANOVA, one-way, followed by Tukey post-test (p < 0.05). p < 0.05 compared to NA, bp < 0.05 compared to DA. There was no statistically significant difference in cholesterol levels between groups.
Corroborating this study, Damasceno, et al. [88], Pepato, et al. [89], and Pepato, et al. [90], when it comes to hypocholesterolemic and antilipidemic, activities results have not been significant. However, De Sousa Lino, et al. [70] verified a reduction of lipid parameters in rats treated with ethanolic, aqueous, and hexane extract of the leaves of B. forficata Link at doses of 200 and 400 mg/kg/day for 7 days.
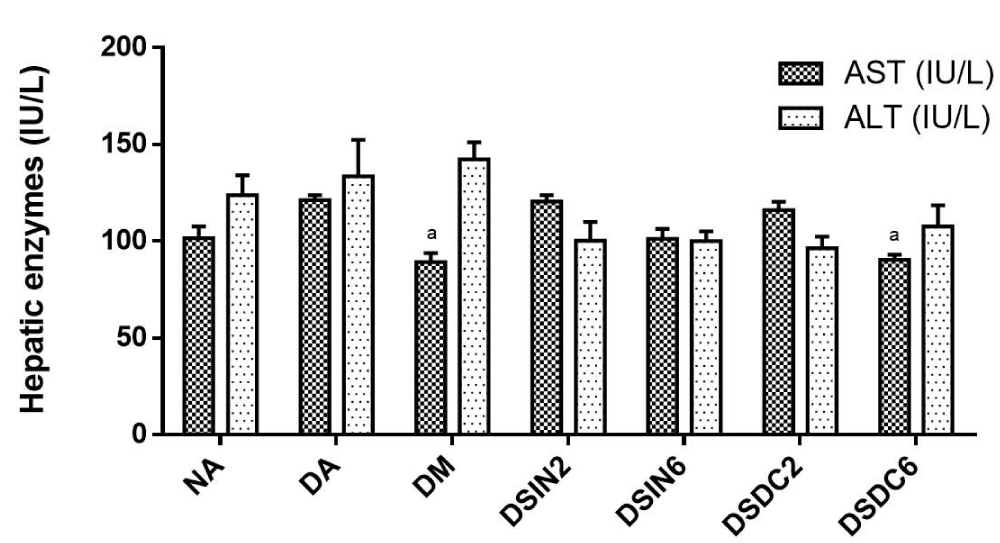
Figure 7 shows the effects of ESIN and ESDC on the hepatic enzymes of diabetic mice. Activities of the liver enzymes aspartate transaminase (AST) and alanine transaminase (ALT) in the groups treated with the extracts did not demonstrate statistically significant changes when compared to non-diabetic mice (NA), displaying an absence of signs of hepatic toxicity. In contrast, Salgueiro, et al. [18], in a study with diabetic rats, found a significant increase in AST when compared to the negative control group, but ALT levels were not altered with B. forficata infusion treatment.
Figure 7: Effects of ESIN and ESDC on hepatic enzymes of diabetic mice. Values represent means ± S.E.M. NA: Negative control; DA: Untreated diabetic animals; DM: Diabetic animals treated with metformin at the dose of 600 mg/kg. DSIN2: Diabetics treated with dry infusion extract at the dose of 200 mg/kg; DSIN6: Diabetic animals treated with dry infusion extract at the dose of 600 mg/kg; DSDC2: Diabetics treated with dry with dry decoction extract at a dose of 200 mg/kg; DSDC6: Diabetic animals treated with dry infusion extract at the dose of 600 mg/kg. Differences in experimental groups were determined by ANOVA, one-way, followed by Tukey post-test (p < 0.05). There was no statistically significant difference between the groups.
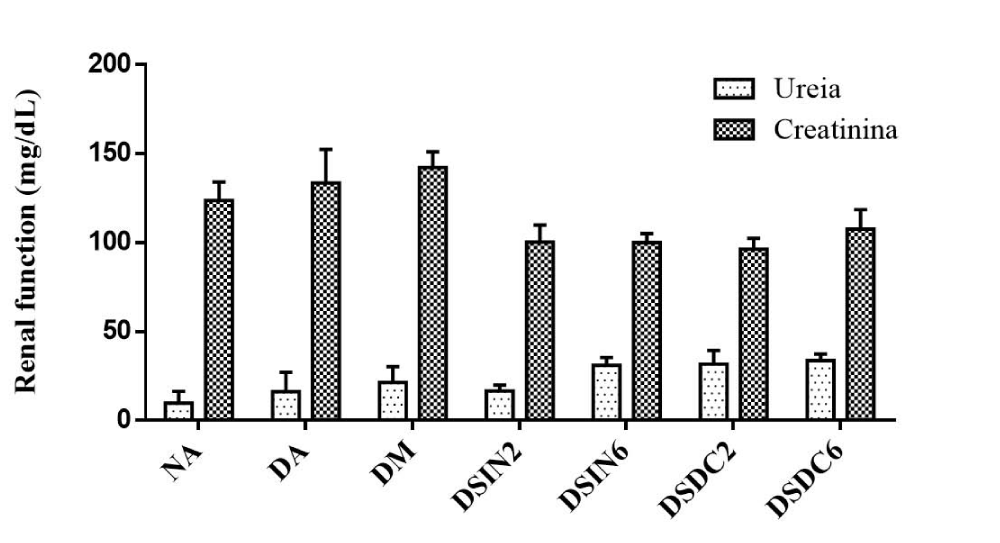
Urea and creatinine levels did not show a significant statistical difference between the treated groups after 28 days of administration of extracts ESIN and ESDC when compared to the negative control group (NA), which shows that the renal function of these animals had no alterations throughout the treatment (Figure 8). However, Mollica, et al. [91], in their study with an infusion from the leaves of plant species Juglans regia L., observed an increase in levels of urea and creatinine in rats treated at a dose of 25 mg/kg, and a decrease at the dose of 50 mg/kg, when compared to the negative control group.
Figure 8: Effect of ESIN and ESDC on renal function in diabetic mice. Values represent means ± S.E.M. Values represent means ± S.E.M. NA: Negative control; DA: Untreated diabetic animals; DM: Diabetic animals treated with metformin at the dose of 600 mg/kg. DSIN2: Diabetics treated with dry infusion extract at the dose of 200 mg/kg; DSIN6: Diabetic animals treated with dry infusion extract at the dose of 600 mg/kg; DSDC2: Diabetics treated with dry decoction extract at a dose of 200 mg/kg; DSDC6: Diabetic animals treated with dry decoction extract at the dose of 600 mg/kg; Differences in experimental groups were determined by ANOVA, one-way, followed by Tukey post-test (p < 0.05). There was no statistically significant difference between the groups.
Effects of ESDC and ESIN on the morphology of the liver, kidneys, and pancreas
Effects of ESDE and ESIN on the morphology of the liver, kidneys, and pancreas of diabetic mice were evaluated through histopathological analysis and are shown in Figures 9-11.
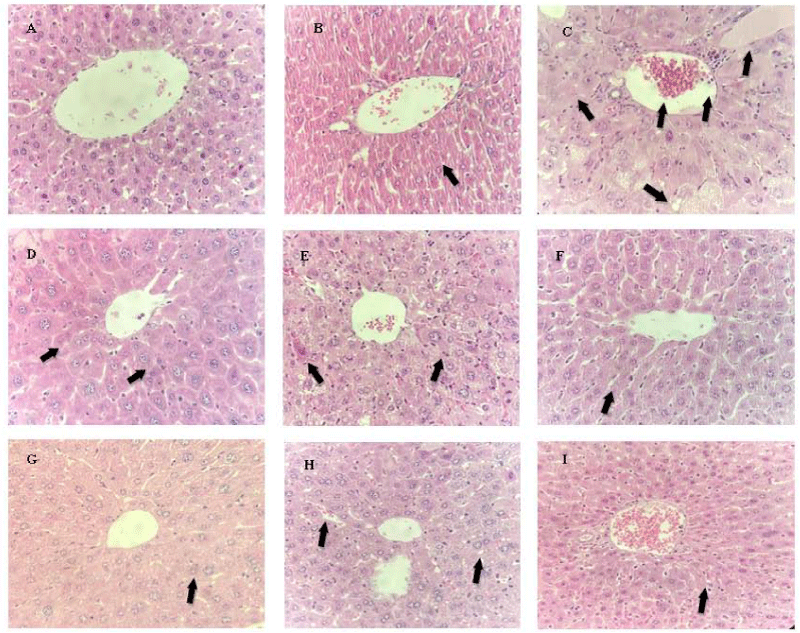
The histopathological profile of the negative control group (NA) displayed a negative control hepatic architecture, with negative control morphology of hepatocytes and hepatic sinusoids organized neatly (Figure 9A). However, group DA exhibited hepatocellular changes, with a decrease between sinusoids (Figure 9B).
Figure 9: Histopathological analysis of the effects of ESIN and ESDC on the liver of Swiss mice. A: NA (Negative control); B: DA (Untreated diabetic animals); C: DM (Diabetic animals treated with metformin at the dose of 600 mg/kg). D: DSIN2 (Diabetic animals treated with 200 mg/kg dry infusion extract); E: DSIN6 (Diabetics animals treated with dry infusion extract at the dose of 600 mg/kg); F: ESIN2 (Animal treated with dry extract infusion at the dose of 2000 mg/kg); G: DSDC2 (Diabetics treated with dry extract of the decoction at the dose of 200 mg/kg); H: DSDC6 (Diabetic animals treated with dry decoction extract at the dose of 600 mg/kg); I: ESDC2 (Standard treated with dry decoction extract at the dose of 2000 mg/kg); Representative photomicrographs of sections stained with hematoxylin and eosin (H & E) X40; Scale bar = 10 μm.
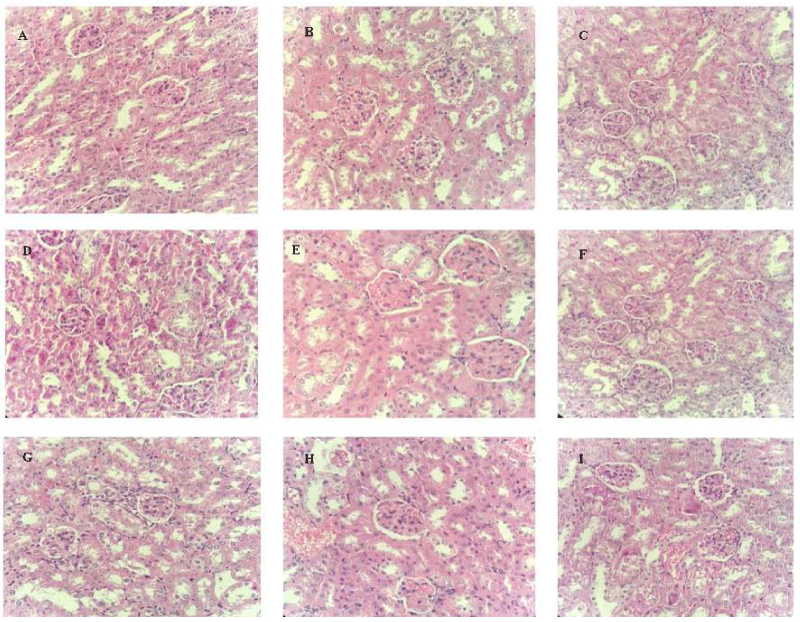
Figure 10: Histopathological analysis of the effects of ESIN and ESDC on the kidneys of Swiss mice. A: NA (Negative control); B: DA (Untreated diabetics); C: DM (Diabetic animals treated with metformin at the dose of 600 mg/kg); D: DSIN2 (Diabetics treated with 200 mg/kg dry infusion extract); E: DSIN6 (Diabetic animals treated with dry infusion extract at the dose of 600 mg/kg); F: ESIN2 (Normally treated with dry extract infusion at the dose of 2000 mg/kg); G: DSDC2 (Diabetic animals treated with dry decoction extract at the dose of 200 mg/kg); H: DSDC6 (Diabetic animals treated with dry decoction extract at the dose of 600 mg/kg); I: ESDC2 (Standard treated with dry decoction extract at the dose of 2000 mg/kg); Representative photomicrographs of sections stained with hematoxylin and eosin (H & E) X40; Scale bar = 10 μm.
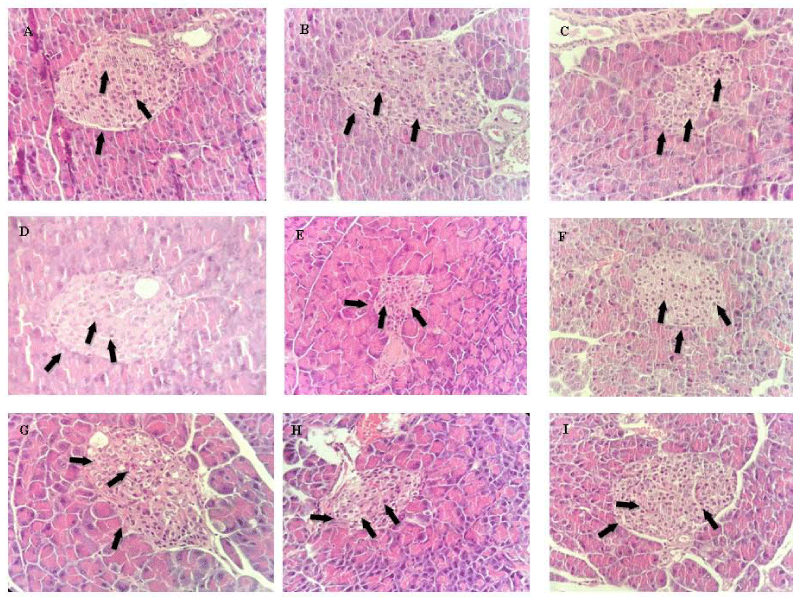
Figure 11: Histopathological analysis of the effects of ESIN and ESDC on the pancreas of Swiss mice. A: NA (Negative control); B: DA (Untreated diabetic animals); C: DM (Diabetic animals treated with metformin at the dose of 600 mg/kg); D: DSIN2 (Diabetic animals treated with 200 mg/kg dry infusion extract); E: DSIN6 (Diabetic animals treated with dry infusion extract at the dose of 600 mg/kg); F: ESIN2 (animals treated with dry infusion extract at the dose of 2000 mg/kg); G: DSDC2 (Diabetic animals treated with dry decoction extract at the dose of 200 mg/kg); H: DSDC6 (Diabetic animals treated with dry decoction extract at the dose of 600 mg/kg); I: ESDC2 (Standard treated with dry decoction extract at the dose of 2000 mg/kg); Representative photomicrographs of sections stained with hematoxylin and eosin (H & E) X40; Scale bar = 10 μm.
In groups DSIN2, DSIN6, and ESIN2, some Kupffer cells were observed around the central vein, the characteristics of which were in agreement with the negative control histology, but there was a mild to moderate loss of liver architecture with some non-radially disposed hepatocytes (Figures 9D, E and F). In the DSDC2, DSDC6, and ESDC2 groups, in addition to what was observed in the other treated groups, fat droplets an increase in vascularization, and the presence of red blood cells in the space of Disse were observed (Figures 9G, H, and I). It is noteworthy that the changes observed in the histopathological analysis did not alter the levels of hepatic enzymes investigated.
Histopathological analysis of the kidneys of diabetic mice showed a renal cortex with renal corpuscles and their glomeruli, as well as proximal and distal tubules, with intact epithelial lining of the glomerular basement membrane and tubular epithelium, besides blood vessels similar to the negative control group (NA), none of the studied groups had alterations in these organs (Figure 10), in consonance with results obtained for urea and creatinine levels, which also did not show changes when compared with the negative control group, confirming that the renal function of these animals was not altered.
The histopathological study of the diabetic mice pancreas revealed that induction of diabetes with STZ caused necrotic alterations and karyolysis, with a decrease of the islets of Langerhans and pancreatic β cells observed in the untreated diabetic (DA) group. The group treated with metformin (DM) had a little more β cells than the DA group but inferior to the NA group. In the DSIN2, DSIN6, and ESIN2 groups, a complete recovery of the pancreatic β cells as well as the pancreatic parenchyma was observed. Groups DSDC2, DSDC6, and ESDC2, have shown to be similar to groups treated using ESIN; however, in group ESDC2, besides an increase in β cells, we also verified a slight decrease in α cells, concerning NA (Figure 11).
Since DM2 is a disorder that arises from the bad functioning of the endocrine pancreas, in which there was a loss or progressive dysfunction of insulin-producing pancreatic β cells, that may be due to a resistance to insulin and glucolipotoxicity, histopathological analyses of this organ are relevant, as already stated by Bakhti, Böttcher, Lickert [92] and Defronzo, et al. [93]. The theory that the main cause of DM2 is related to secretion and action of insulin has been recently confirmed by Chen, et al. [94], although other factors are also relevant, such as the activation of intracellular stress response pathways and inter-organ communication networks mediated by inflammatory molecules (cytokines) and peptide hormones. As there is still no treatment that can stop or revert the progress of this disease, results from this study are promising, since, as observed, the ESIN and ESDC extracts promoted the recovered pancreatic β cells, constituting possible alternatives for the treatment of DM2.
Alves, et al. [95] affirmed that research on natural active substances has grown over the past years, focused on the search for novel drugs with higher therapeutic activity, lower toxicity, better biocompatibility, and greater accessibility to the population who, due to cultural aspects, well accepts phytotherapy. The market of therapeutic products made from bioactive compounds present in medicinal plants presents a good perspective. Thus, studies such as this are relevant for the development of safe natural medicines that are efficient in the treatment of diabetes mellitus.
Chromatographic analysis has shown that the nanoencapsulation technique has preserved polyphenols, flavonoids, and their O-glycosylated derivatives in the dried infusion and decoction extracts of Bauhinia forficata Link, besides a significant antioxidant capacity, are potentially responsible for its beneficial effect in the regeneration of pancreatic β cells, with a decrease in blood glucose levels in diabetic mice superior to that of metformin. During the acute toxicity assays, extracts demonstrated genotoxic effects on bone marrow and on the liver, which were repaired after 14 days of treatment. However, these effects were not observed in the pancreas and peripheral blood. Nanoencapsulated extracts promoted the regeneration of pancreatic β cells, decreasing blood glucose levels in diabetic mice, with a superior beneficial effect to metformin, showing therapeutic potential for application in pharmaceutical formulations with antidiabetic activity.
Credit author statement
Bárbara Verônica Cardoso de Souza: Conceptualization, Writing - Original Draft, Writing - Review and Editing, formal analysis, data curation, responses. Alessandra Braga Ribeiro: Data Curation, methodology. Rita de Cássia Meneses Oliveira: Methodology, validation, resources. Julianne Viana Freire Portela: Research, review. Ana Amélia de Carvalho Melo Cavalcante: Writing - Review, methodology, data curation, formal analysis. Esmeralda Maria Lustosa Barros: Research. Luís Felipe Lima Matos: Research. Tarsia Giabardo Alves: Research. Maria do Carmo de Carvalho e Martins: Conceptualization, methodology, formal analysis. Lívio César Cunha Nunes: Project management – supervision, conceptualization, methodology, review.
- Chatterjee S, Khunti K, Davies MJ. Diabetes type 2. Lancet. 2017;389(10085):2239-2251. Available from: https://doi.org/10.1016/s0140-6736(17)30058-2.
- Li C, Bishop TRP, Imamura F, Sharp SJ, Pearce M, Brage S, et al. Meat consumption and incident type 2 diabetes: an individual-participant federated meta-analysis of 1·97 million adults with 100 000 incident cases from 31 cohorts in 20 countries. Lancet Diabetes Endocrinol. 2024;12(9):619-630. Available from: https://doi.org/10.1016/s2213-8587(24)00179-7.
- Ajuwon OR, Ayeleso AO, Adefolaju GA. The Potential of South African Herbal Tisanes, Rooibos and Honeybush in the Management of Type 2 Diabetes Mellitus. Molecules. 2018;23(12):3207. Available from: https://doi.org/10.3390/molecules23123207.
- Kangralkar VA, Patil SD, Bandivadekar RM. Oxidative stress and diabetes: a review. Int J Pharm Appl. 2010;1(1):38-45. Available from: http://bipublication.com/files/phv1I120106.pdf.
- Moussa SA. Oxidative stress in diabetes mellitus. Rom J Biophys. 2008;18(3):225-236. Available from: https://www.rjb.ro/articles/214/samou.pdf.
- Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J. 2016;24(5):547-553. Available from: https://doi.org/10.1016/j.jsps.2015.03.013.
- Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11(4):1365-402. Available from: https://doi.org/10.3390/ijms11041365.
- Walton EL. Oxidative stress and diabetes: Glucose response in the cROSsfire. Biomed J. 2017 ;40(5):241-244. Available from: https://doi.org/10.1016/j.bj.2017.10.001.
- Zhu D, Zhang X, Niu Y, Diao Z, Ren B, Li X, et al. Cichoric acid improved hyperglycaemia and restored muscle injury via activating antioxidant response in MLD-STZ-induced diabetic mice. Food Chem Toxicol. 2017;107(Pt A):138-149. Available from: https://doi.org/10.1016/j.fct.2017.06.041.
- Trojan-Rodrigues M, Alves TL, Soares GL, Ritter MR. Plants used as antidiabetics in popular medicine in Rio Grande do Sul, southern Brazil. J Ethnopharmacol. 2012;139(1):155-63. Available from: https://doi.org/10.1016/j.jep.2011.10.034
- Alkhalidy H, Moore W, Wang Y, Luo J, McMillan RP, Zhen W, et al. The Flavonoid Kaempferol Ameliorates Streptozotocin-Induced Diabetes by Suppressing Hepatic Glucose Production. Molecules. 2018;23(9):2338. Available from: https://doi.org/10.3390/molecules23092338
- Ecker A, Gonzaga TKSDN, Seeger RL, Santos MMD, Loreto JS, Boligon AA, et al. High-sucrose diet induces diabetic-like phenotypes and oxidative stress in Drosophila melanogaster: Protective role of Syzygium cumini and Bauhinia forficata. Biomed Pharmacother. 2017;89:605-616. Available from: https://doi.org/10.1016/j.biopha.2017.02.076
- Da Cunha AM, Menon S, Menon R, Couto AG, Bürger C, Biavatti MW. Hypoglycemic activity of dried extracts of Bauhinia forficata Link. Phytomedicine. 2010;17(1):37-41. Available from: https://doi.org/10.1016/j.phymed.2009.06.007.
- Jorge AP, Horst H, de Sousa E, Pizzolatti MG, Silva FR. Insulinomimetic effects of kaempferitrin on glycaemia and on 14C-glucose uptake in rat soleus muscle. Chem Biol Interact. 2004;149(2-3):89-96. Available from: https://doi.org/10.1016/j.cbi.2004.07.001
- Khalil NM, Pepato MT, Brunetti IL. Free radical scavenging profile and myeloperoxidase inhibition of extracts from antidiabetic plants: Bauhinia forficata and Cissus sicyoides. Biol Res. 2008;41(2):165-171. Available from: http://dx.doi.org/10.4067/S0716-97602008000200006.
- Pizzolatti MG, Cunha Jr A, Szpoganicz B, Sousa ED, Braz-Filho R, Schripsema J. Flavonoids glycosides from leaves and flowers of Bauhinia forficata (Leguminosae). Quim Nova. 2003;26(4):466-469. Available from: https://doi.org/10.1590/S0100-40422003000400003.
- Salatino A, Blatt CT, Santos DYD, Vaz AM. Foliar flavonoids of nine species of Bauhinia. Braz J Bot. 1999;22(1):17-20. Available from: https://doi.org/10.1590/S0100-84041999000100003.
- Salgueiro AC, Folmer V, da Silva MP, Mendez AS, Zemolin AP, Posser T, et al. Effects of Bauhinia forficata Tea on Oxidative Stress and Liver Damage in Diabetic Mice. Oxid Med Cell Longev. 2016;2016:8902954. Available from: https://doi.org/10.1155/2016/8902954.
- da Silva KL, Biavatti MW, Leite SN, Yunes RA, Delle Monache F, Cechinel Filho V. Phytochemical and pharmacognositc investigation of Bauhinia forficata Link (Leguminosae). Z Naturforsch C J Biosci. 2000;55(5-6):478-80. Available from: https://doi.org/10.1515/znc-2000-5-627.
- de Sousa E, Zanatta L, Seifriz I, Creczynski-Pasa TB, Pizzolatti MG, Szpoganicz B, Silva FR. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(alpha)-dirhamnoside from Bauhinia forficata leaves. J Nat Prod. 2004;67(5):829-32. Available from: https://doi.org/10.1021/np030513u.
- Tzeng YM, Chen K, Rao YK, Lee MJ. Kaempferitrin activates the insulin signaling pathway and stimulates secretion of adiponectin in 3T3-L1 adipocytes. Eur J Pharmacol. 2009;607(1-3):27-34. Available from: https://doi.org/10.1016/j.ejphar.2009.01.023.
- Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab (Lond). 2015;12(1):60. Available from: https://doi.org/10.1186/s12986-015-0057-7.
- Alonso-Castro AJ, Ortiz-Sánchez E, García-Regalado A, Ruiz G, Núñez-Martínez JM, González-Sánchez I, et al. Kaempferitrin induces apoptosis via intrinsic pathway in HeLa cells and exerts antitumor effects. J Ethnopharmacol. 2013;145(2):476-89. Available from: https://doi.org/10.1016/j.jep.2012.11.016.
- Cechinel-Zanchett CC, Boeing T, Somensi LB, et al. Flavonoid-rich fraction of Bauhinia forficata Link leaves prevents the intestinal toxic effects of irinotecan chemotherapy in IEC-6 cells and in mice. Phytother Res. 2019;33:90-106. Available from: https://doi.org/10.1002/ptr.6202.
- Miceli N, Buongiorno LP, Celi MG, Cacciola F, Dugo P, Donato P, et al. Role of the flavonoid-rich fraction in the antioxidant and cytotoxic activities of Bauhinia forficata Link. (Fabaceae) leaves extract. Nat Prod Res. 2016;30(11):1229-39. Available from: https://doi.org/10.1080/14786419.2015.1050671.
- De Pontes MAN, De Lima DS, De Oliveira HMBF, De Oliveira Filho AA. Bauhinia forficata L. and its hypoglycemic action. Arch Health Investig. 2017;6(11). Available from: https://doi.org/10.21270/archi.v6i11.2244.
- de Souza BVC, Moreira Araújo RSDR, Silva OA, Faustino LC, Gonçalves MFB, Dos Santos ML, et al. Bauhinia forficata in the treatment of diabetes mellitus: a patent review. Expert Opin Ther Pat. 2018;28(2):129-138. Available from: https://doi.org/10.1080/13543776.2018.1409208.
- Arpagaus C, Collenberg A, Rütti D, Assadpour E, Jafari SM. Nano spray drying for encapsulation of pharmaceuticals. Int J Pharm. 2018;546(1-2):194-214. Available from: https://doi.org/10.1016/j.ijpharm.2018.05.037.
- Cechinel-Zanchett CC, De Andrade SF, Cechinel-Filho V. Ethnopharmacological, phytochemical, pharmacological and toxicological aspects of Bauhinia forficata: A mini-review covering the last five years. Nat Prod Commun. 2018;13(7):911-916. Available from: https://doi.org/10.1177/1934578X1801300732.
- Funes-Rivera S, Kennedy ML, Galeano AK, Torres PMF, Campuzano-Bublitz MA. Methanol extract of Bauhinia forficata leaves reduced serum creatinine level and prevented the elevation of hepatic enzymes in mice exposed to gentamicin and acetaminophen: an exploratory study. Biomed Biopharm Res. 2023;20(1):64-82. Available from: http://dx.doi.org/10.19277/bbr.20.1.311.
- Chan K, Shaw D, Simmonds MS, Leon CJ, Xu Q, Lu A, et al. Good practice in reviewing and publishing studies on herbal medicine, with special emphasis on traditional Chinese medicine and Chinese materia medica. J Ethnopharmacol. 2012;140(3):469-75. Available from: doi: https://doi.org/10.1016/j.jep.2012.01.038.
- Menezes FDS, Minto ABM, Ruela HS, Kuster RM, Sheridan H, Frankish N, et al. Hypoglycemic activity of two Brazilian Bauhinia species: Bauhinia forficata L. and Bauhinia monandra Kurz. Rev Bras Farmacogn. 2007;17(1):8-13. Available from: https://doi.org/10.1590/S0102-695X2007000100003.
- Rolim Neto P, Lyra M, Silva R, Nunes L, Alves M, Rolim L, et al. Effervescent pharmaceutical composition and use of ethanolic extract of Bauhinia forficat. BR 10 2013 024150 4 A2. 2015. Available from: https://repositorio.ufpe.br/handle/123456789/35645.
- Verônica Cardoso de Souza B, de Morais Sousa M, Augusto Gasparotto Sattler J, Cristina Sousa Gramoza Vilarinho Santana A, Bruno Fonseca de Carvalho R, de Sousa Lima Neto J, et al. Nanoencapsulation and bioaccessibility of polyphenols of aqueous extracts from Bauhinia forficata link. Food Chem (Oxf). 2022;5:100144. Available from: https://doi.org/10.1016%2Fj.fochms.2022.100144.
- Swain T, Hills WE. The phenolic constituents of Prunus domestica. I- quantitative analysis of phenolics constituents. J Sci Food Agric. 1959;19:63-68. Available from: https://ui.adsabs.harvard.edu/link_gateway/1959JSFA...10...63S/doi:10.1002/jsfa.2740100110
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555-559. Available from: https://doi.org/10.1016/S0308-8146(98)00102-2.
- Kim DO, Lee KW, Lee HJ, Lee CY. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J Agric Food Chem. 2002 Jun 19;50(13):3713-7. Available from: https://doi.org/10.1021/jf020071c.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999 May;26(9-10):1231-7. Available from: https://doi.org/10.1016/s0891-5849(98)00315-3.
- Arnous A, Makris DP, Kefalas P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J Food Compos Anal. 2002;15:655-665. Available from: https://doi.org/10.1006/jfca.2002.1070.
- Prior RL, Hoang H, Gu L, Wu X, Bacchiocca M, Howard L, et al. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J Agric Food Chem. 2003 May 21;51(11):3273-9. Available from: https://doi.org/10.1021/jf0262256.
- Dávalos A, Gómez-Cordovés C, Bartolomé B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J Agric Food Chem. 2004 Jan 14;52(1):48-54. Available from: https://doi.org/10.1021/jf0305231.
- Cuyckens F, Claeys M. Mass spectrometry in the structural analysis of flavonoids. J Mass Spectrom. 2004 Jan;39(1):1-15. Available from: https://doi.org/10.1002/jms.585.
- Farag MA, Sakna ST, El-Fiky NM, Shabana MM, Wessjohann LA. Phytochemical, antioxidant and antidiabetic evaluation of eight Bauhinia L. species from Egypt using UHPLC-PDA-qTOF-MS and chemometrics. Phytochemistry. 2015 Nov;119:41-50. Available from: https://doi.org/10.1016/j.phytochem.2015.09.004.
- Ferreres F, Gil-Izquierdo A, Vinholes J, Silva ST, Valentão P, Andrade PB, et al. Bauhinia forficata Link authenticity using flavonoids profile: relation with their biological properties. Food Chem. 2012 Sep 15;134(2):894-904. Available from: https://doi.org/10.1016/j.foodchem.2012.02.201.
- Diderich R. The OECD chemicals program. In: Risk Assessment of Chemicals. Dordrecht: Springer. 2007; 623-638. Available from: http://dx.doi.org/10.1007/978-1-4020-6102-8_16.
- Malone MH. The pharmacological evaluation of natural products--general and specific approaches to screening ethnopharmaceuticals. J Ethnopharmacol. 1983 Aug;8(2):127-47. Available from: https://doi.org/10.1016/0378-8741(83)90050-8.
- Cunha LC, Azeredo FS, Mendonça AC, Vieira MS, Pucci LL, Valadares MC, et al. Assessment of acute and subacute toxicity, in rats, of the ethanolic extract of the leaves and latex of Synadenium umbellatum Pax. Rev Bras Farmacogn. 2009;19(2):403-411. Available from: https://doi.org/10.1590/S0102-695X2009000300012.
- Brito AS. Manual de ensaios toxicológicos in vivo. Campinas: UNICAMP; 1994. 122 p. Available from: https://pesquisa.bvsalud.org/portal/resource/pt/lil-641100.
- da Silva AP, Oliveira GL, Medeiros SC, Sousa AM, Lopes Lda S, David JM, et al. Pre-clinical toxicology of garcinielliptone FC, a tautomeric pair of polyprenylated benzophenone, isolated from Platonia insignis Mart seeds. Phytomedicine. 2016 May 15;23(5):477-82. Available from: https://doi.org/10.1016/j.phymed.2016.02.013.
- Speit G, Rothfuss A. The comet assay: a sensitive genotoxicity test for the detection of DNA damage and repair. Methods Mol Biol. 2012;920:79-90. Available from: https://doi.org/10.1007/978-1-61779-998-3_6
- Ferreira FAG, Silva FC, Carvalho CM, Costa JC, Ferreira JMR. Hematological and plasma biochemical profile of mice after ingestion of the Arrabidaea chica plant. Scientia Plena. 2016;12(9). Available from: https://doi.org/10.14808/sci.plena.2016.091005.
- Fröde TS, Medeiros YS. Animal models to test drugs with potential antidiabetic activity. J Ethnopharmacol. 2008 Jan 17;115(2):173-183. Available from: https://doi.org/10.1016/j.jep.2007.10.038.
- Matteucci E, Giampietro O. Proposal open for discussion: defining agreed diagnostic procedures in experimental diabetes research. J Ethnopharmacol. 2008 Jan 17;115(2):163-172. Available from: https://doi.org/10.1016/j.jep.2007.08.040.
- Pereira ACS, Ribeiro GE, Souza LCR, Rufino LRA, Cabral ISR, Boriollo MFG, et al. Biologic activity of the hydroalcoholic extract of Bauhinia forficata Link on Herpetomonas samuelpessoai (Galvão.) Roitman. Rev Bras Plantas Med. 2014;16(3):585-592. Available from: https://doi.org/10.1590/1983-084X/13_093.
- Pan Y, Wang C, Chen Z, Li W, Yuan G, Chen H. Physicochemical properties and antidiabetic effects of a polysaccharide from corn silk in high-fat diet and streptozotocin-induced diabetic mice. Carbohydr Polym. 2017 May 15;164:370-378. Available from: https://doi.org/10.1016/j.carbpol.2017.01.092.
- Ribeiro AFC, Telles TC, Ferraz VP, Souza-Fagundes EM, Cassali GD, Carvalho AT, et al. Effect of Arrabidaea chica extracts on the Ehrlich solid tumor development. Rev Bras Farmacogn. 2012;22(2):364-373. Available from: https://doi.org/10.1590/S0102-695X2011005000225.
- Li PB, Lin WL, Wang YG, Peng W, Cai XY, Su WW. Antidiabetic activities of oligosaccharides of Ophiopogonis japonicus in experimental type 2 diabetic rats. Int J Biol Macromol. 2012 Dec;51(5):749-55. Available from: https://doi.org/10.1016/j.ijbiomac.2012.07.007.
- Wang C, Chen Z, Pan Y, Gao X, Chen H. Anti-diabetic effects of Inonotus obliquus polysaccharides-chromium (III) complex in type 2 diabetic mice and its sub-acute toxicity evaluation in normal mice. Food Chem Toxicol. 2017 Oct;108(Pt B):498-509. Available from: https://doi.org/10.1016/j.fct.2017.01.007.
- Franco RR, da Silva Carvalho D, de Moura FBR, Justino AB, Silva HCG, Peixoto LG, et al. Antioxidant and anti-glycation capacities of some medicinal plants and their potential inhibitory against digestive enzymes related to type 2 diabetes mellitus. J Ethnopharmacol. 2018 Apr 6;215:140-146. Available from: https://doi.org/10.1016/j.jep.2017.12.032.
- Bjørklund G, Chirumbolo S. Role of oxidative stress and antioxidants in daily nutrition and human health. Nutrition. 2017 Jan;33:311-321. Available from: https://doi.org/10.1016/j.nut.2016.07.018.
- Saliu JA, Ademiluyi AO, Akinyemi AJ, Oboh G. Propriedades antidiabetes e anti-hipertensoras in vitro de extratos fenólicos de folhas amargas (Vernonia amygdalina Del.). J Food Biochem. 2012;36(5):569-76.
- Da Silva KL, Filho VC. Plants of the genus Bauhinia: chemical composition and pharmacological potential Quím Nova. 2002;25(3):449-54. Available from: https://doi.org/10.1590/S0100-40422002000300018
- Filho VC. Chemical composition and biological potential of plants from the genus Bauhinia. Phytother Res. 2009;23(10):1347-54. Available from: https://doi.org/10.1002/ptr.2756
- Farias LS, Mendez AS. LC/ESI-MS method applied to characterization of flavonoids glycosides in B. forficata subsp. pruinosa. Quím Nova. 2014;37(3):483-6. Available from: https://doi.org/10.5935/0100-4042.20140069
- Santos M, Fortunato RH, Spotorno VG. Analysis of flavonoid glycosides with potential medicinal properties on Bauhinia uruguayensis and Bauhinia forficata subspecies pruinosa. Nat Prod Res. 2018;33:1-5. Available from: http://dx.doi.org/10.1080/14786419.2018.1460826
- Düsman E, Almeida IVD, Coelho AC, Balbi TJ, Düsman Tonin LT, Vicentini VEP. Antimutagenic effect of medicinal plants Achillea millefolium and Bauhinia forficata in vivo. Evid Based Complement Alternat Med. 2013;2013:893050. Available from: https://doi.org/10.1155/2013/893050
- Pepato MT, Baviera AM, Vendramini RC, Brunetti IL. Evaluation of toxicity after one-months treatment with Bauhinia forficata decoction in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2004;4(1):7. Available from: https://doi.org/10.1186/1472-6882-4-7
- Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, Khetani VA. A 90-day oral gavage toxicity study of p-methylphenidate and d,l-methylphenidate in Sprague Dawley rats. Toxicology. 2002;179:183-196. Available from: https://doi.org/10.1016/s0300-483x(02)00338-4
- Lu L, Fan Y, Yao W, Xie W, Guo J, Yan Y, et al. Safety assessment of the fermented Phylloporia ribis (Lonicera japônica Thunb.) mycelia by oral acute toxicity study in mice and 90-day feeding study in rats. Food Chem Toxicol. 2014;69:18-24. Available from: https://doi.org/10.1016/j.fct.2014.03.044
- De Sousa Lino C, Diógenes JPL, Pereira BA, Faria RAPG, Neto MA, Alves RS, Viana GSB. Antidiabetic activity of Bauhinia forficata extracts in alloxan-diabetic rats. Biol Pharm Bull. 2004;27(1):125-127. Available from: https://doi.org/10.1248/bpb.27.125
- Varela-Barca F, Agnez-Lima LF, De Medeiros SRB. Base excision repair pathway is involved in the repair of lesions generated by flavonoid-enriched fractions of pepper tree (Schinus terebinthifolius, Raddi) stem bark. Environ Mol Mutagen. 2007;48(8):672-681. Available from: https://doi.org/10.1002/em.20334
- Hodek P, Trefil P, Stiborová M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact. 2002;139(1):1-21. Available from: https://doi.org/10.1016/s0009-2797(01)00285-x
- Skibola CF, Smith MT. Potential health impacts of excessive flavonoid intake. Free Radic Biol Med. 2000;29(3-4):375-383. Available from: https://doi.org/10.1016/s0891-5849(00)00304-x
- Okonkwo COJ, Ehileboh AD, Nwobodo E, Dike CC. The effects of acute gasoline vapor inhalation on some hematological indices of albino Wistar rats. J Acute Dis. 2016;5(2):123-125. Available from: http://dx.doi.org/10.1016/j.joad.2015.11.005
- Cerqueira FM, De Medeiros MHG, Augusto O. Dietary antioxidants: controversies and perspectives. Quím Nova. 2007;30(2):441. Available from: https://doi.org/10.1590/S0100-40422007000200036
- Martínezmartínez BB, Pereira ACC, Muzetti JH, De Paiva Telles F, Mundim FGL, Teixeira MA. Bauhinia forficata and glucocorticoid-induced insulin resistance. Int J Sci Res. 2018;6(10).
- Cobble ME, Peters AL. Clinical practice in type 2 diabetes: After metformin and lifestyle, then what? J Fam Pract. 2009;58(11 Suppl Clinical): S7-14. Available from: https://pubmed.ncbi.nlm.nih.gov/19891948/
- Ahmed AS, Elgorashi EE, Moodley N, Mcgaw LJ, Naidoo V, Eloff JN. The antimicrobial, antioxidative, anti-inflammatory activity and cytotoxicity of different fractions of four South African Bauhinia species used traditionally to treat diarrhea. J Ethnopharmacol. 2012;143(3):826-839. Available from: https://doi.org/10.1016/j.jep.2012.08.004
- Cazarolli LH, Folador P, Moresco HH, Brighente IMC, Pizzolatti MG, Silva FRMB. Mechanism of action of the stimulatory effect of apigenin-6-C-(2 ″-O-α-l-rhamnopyranosyl)-β-l-fucopyranoside on 14C-glucose uptake. Chem Biol Interact. 2009;179(2-3):407-412. Available from: https://doi.org/10.1016/j.cbi.2008.11.012
- Cazarolli LH, Pereira DF, Kappel VD, Folador P, Figueiredo MDSRB, Pizzolatti MG, et al. Insulin signaling: a potential signaling pathway for the stimulatory effect of kaempferitrin on glucose uptake in skeletal muscle. Eur J Pharmacol. 2013;712(1-3):1-7. Available from: https://doi.org/10.1016/j.ejphar.2013.02.029
- Ren J, Lu Y, Qian Y, Chen B, Wu T, Ji G. Recent progress regarding kaempferol for the treatment of various diseases. Exp Ther Med. 2019;18(4):2759-76. Available from: https://doi.org/10.3892/etm.2019.7886
- Sharma B, Balomajumder C, Roy P. Hypoglycemic and hypolipidemic effects of flavonoid-rich extract from Eugenia jambolana seeds on streptozotocin-induced diabetic rats. Food Chem Toxicol. 2008;46(7):2376-2383. Available from: https://doi.org/10.1016/j.fct.2008.03.020
- Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications-a review. Nutr J. 2014;13:17. Available from: https://doi.org/10.1186/1475-2891-13-17
- Mirza AZ, Althagafi II, Shamshad H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur J Med Chem. 2019;166:502-513. Available from: https://doi.org/10.1016/j.ejmech.2019.01.067
- Tonelli CA, de Oliveira SQ, da Silva Vieira AA, Biavatti MW, Ritter C, Reginatto FH, et al. Clinical efficacy of capsules containing standardized extract of Bauhinia forficata Link (pata-de-vaca) as adjuvant treatment in type 2 diabetes patients: A randomized, double-blind clinical trial. J Ethnopharmacol. 2022;282:114616. Available from: https://doi.org/10.1016/j.jep.2021.114616
- Xu H, Luo J, Huang J, Wen Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine. 2018;97(19):e0686. Available from: https://doi.org/10.1097/md.0000000000010686
- Volpato GT, Damasceno DC, Rudge MVC, Padovani CR, Calderon IDMP. Effect of Bauhinia forficata aqueous extract on the maternal-fetal outcome and oxidative stress biomarkers of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2008;116(1):131-137. Available from: https://doi.org/10.1016/j.jep.2007.11.013
- Damasceno DC, Volpato GT, Calderon IDMP, Aguilar R, Rudge MC. Effect of Bauhinia forficata extract in diabetic pregnant rats: maternal repercussions. Phytomedicine. 2004;11(2/3):196-201. Available from: https://doi.org/10.1078/0944-7113-00348
- Pepato MT, Conceição CQ, Gutierres VO, Vendramini RC, Souza CRF, Oliveira WP, Brunetti IL. Evaluation of the spouted bed dried leaf extract of Bauhinia forficata for the treatment of experimental diabetes in rats. Afr J Biotechnol. 2010;9(42):7157-64. Available from: http://hdl.handle.net/11449/226097.
- Pepato MT, Keller EH, Baviera AM, Kettelhut IC, Vendramini RC, Brunetti IL. Anti-diabetic activity of Bauhinia forficata decoction in streptozotocin-diabetic rats. J Ethnopharmacol. 2002;81(2):191-197. Available from: https://doi.org/10.1016/s0378-8741(02)00075-2
- Mollica A, Zengin G, Locatelli M, Stefanucci A, Macedonio G, Bellagamba G, et al. An assessment of the nutraceutical potential of Juglans regia L. leaf powder in diabetic rats. Food Chem Toxicol. 2017;107:554-564. Available from: https://doi.org/10.1016/j.fct.2017.03.056
- Bakhti M, Böttcher A, Lickert H. Modelling the endocrine pancreas in health and disease. Nat Rev Endocrinol. 2019 Mar;15(3):155-171. Available from: https://doi.org/10.1038/s41574-018-0132-z
- Defronzo RA, Ferrannini E, Groop L, Henrique RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers. 2015;1:15019. Available from: https://doi.org/10.1038/nrdp.2015.19
- Chen L, Gnanaraj C, Arulselvan P, El-Seedi H, Teng H. A review on advanced nanoencapsulation technology to enhance bioavailability of phenolic compounds: based on its activity in the treatment of type 2 diabetes. Trends Food Sci Technol. 2019;85:149-162. Available from: https://doi.org/10.1016/j.tifs.2018.11.026
- Alves EP, Freires IA, Rosalen PL, Ruiz AL, Granville-Garcia AF, Godoy GP, et al. Antimicrobial and antiproliferative activity of Bauhinia forficata Link and Cnidoscolus quercifolius extracts commonly used in folk medicine. J Contemp Dent Pract. 2017;18(8):635-640. Available from: https://doi.org/10.5005/jp-journals-10024-2098