More Information
Submitted: January 07, 2025 | Approved: January 23, 2025 | Published: January 24, 2025
How to cite this article: Menu WMS. In vitro, Anti-oxidant, and Anti-inflammatory Activity of Kalanchoe pinnata. Arch Pharm Pharma Sci. 2025; 9(1): 001-008. Available from:
https://dx.doi.org/10.29328/journal.apps.1001064
DOI: 10.29328/journal.apps.1001064
Copyright License: © 2025 Menu WMS. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction inany medium, provided the original work is properly cited.
Keywords: Kalanchoe pinnata; Antioxidant activity; Anti-inflammatory activity; Phytochemicals; Traditional medicine
In vitro, Anti-oxidant, and Anti-inflammatory Activity of Kalanchoe pinnata
Wijeratne Mudiyanselage Swarna Menu*
Department of Biomedical Science, Faculty of Health Sciences, KAATSU International University, No. 249/1, Malabe Rd., Thalangama North, Koswatta, Battaramulla, Sri Lanka
*Address for Correspondence: Wijeratne Mudiyanselage Swarna Menu, Department of Biomedical Science, Faculty of Health Sciences, KAATSU International University, No. 249/1, Malabe Rd., Thalangama North, Koswatta, Battaramulla, Sri Lanka, Email: [email protected]
Kalanchoe pinnata is a widely recognized medicinal plant known for its antioxidant and anti-inflammatory properties. This study explores its in-vitro antioxidant and anti-inflammatory activities, highlighting its potential for pharmaceutical and biomedical applications. The research innovatively assesses its bioactive components using DPPH radical scavenging, nitric oxide inhibition assays, and phenolic content analysis. Results demonstrated significant antioxidant activity with IC50 values comparable to ascorbic acid, along with notable anti-inflammatory effects via nitric oxide inhibition. These findings emphasize Kalanchoe pinnata’s potential as a source for developing antioxidant and anti-inflammatory therapeutics. Further investigation into bioactive compound isolation and mechanistic pathways is recommended to clarify its pharmacological efficacy.
Medicinal plants have long been essential in traditional medicine, offering a wealth of bioactive compounds with therapeutic potential. Kalanchoe pinnata, or “miracle leaf,” has garnered attention for its significant antioxidant and anti-inflammatory properties [1-4]. Native to Madagascar and now prevalent in tropical and subtropical regions, Kalanchoe pinnata has historical use in treating wounds, infections, and inflammatory conditions, highlighting its ethnomedicinal importance [1,2] (Figure 1).
Figure 1: Kalanchoe pinnata (“miracle leaf”), a perennial succulent plant recognized for its medicinal properties, particularly its antioxidant and anti-inflammatory potential.
The rising incidence of chronic diseases such as cardiovascular disorders, diabetes, and cancer underscores the vital roles of oxidative stress and inflammation in disease progression [5]. Natural antioxidants and anti-inflammatory agents from plants have emerged as safer alternatives to synthetic drugs, which often carry adverse side effects [6,7]. Although previous studies have identified flavonoids, phenols, and triterpenoids in Kalanchoe pinnata, comprehensive assessments of its antioxidant and anti-inflammatory properties remain scarce [4,8,9].
Inflammation is a complex physiological process mediated by various signaling molecules essential for host defense. Damage to cell membranes causes phospholipase A2 to degrade phospholipids, releasing arachidonic acid. This initiates the formation of leukotrienes through the lipoxygenase pathway and prostaglandins via the cyclooxygenase pathway, particularly COX-2 [10,11]. Prostaglandins from COX-2 pathways are significant inflammation mediators, linked to diseases like asthma, arthritis, and cancer [11].
The rising interest in medicinal plants like Kalanchoe pinnata is due to their potential as safer alternatives to conventional therapies. Phytochemicals such as flavonoids, terpenes, alkaloids, and phenolics in K. pinnata exhibit significant pharmacological properties [1,2,4]. Known as “Akkapana” in Sri Lanka, Kalanchoe pinnata is a succulent perennial plant commonly found in gardens and wild in hilly regions [1]. Traditionally, its aqueous leaf extract has been used to treat kidney stones, gastric ulcers, and pulmonary infections [1,12]. While it poses ecological concerns as an invasive species in some areas, its pharmacological potential is noteworthy [1,12].
This study aims to systematically examine the in-vitro antioxidant and anti-inflammatory activities of Kalanchoe pinnata. Utilizing innovative methods such as DPPH radical scavenging [13], nitric oxide inhibition assays [20], and phenolic content quantification, the research seeks to fill knowledge gaps and support the development of natural therapeutic agents [13,14]. By building on prior findings, the study underscores Kalanchoe pinnata’s value as a source of bioactive compounds for combating oxidative stress and inflammation [1,2, 4,8, 9,15].
Preparation of the extract of Kalanchoe pinnata
The plant was extracted by using maceration as the extraction method. After collecting the fresh leaves of Kalanchoe pinnata they were washed properly using clean water and then dried using air drying (Figure 2). After the drying process, the fresh leaves were cut into small pieces (about 1 cm). Then 100 g of pieces were measured using a scale. After being measured they were chopped until we could get the pulp (Figure 3). After that, the fresh pulp was extracted with 99% acetone (Figure 4). The ratio of Acetone: to plant material should be 2: 1 (v/w). The extract was filtered after 72 hours using filter papers. The filtrate was evaporated to collect a dry powder (Figure 5). After the day, the dry powder was collected from the leaves of Kalanchoe pinnata (Figure 6).
Figure 2: Preparation of Kalanchoe pinnata leaves for experimental procedures; fresh leaves were collected, thoroughly washed with clean water to remove debris, and then air-dried under controlled conditions to preserve bioactive compounds. This preparation ensures the integrity and consistency of samples for subsequent assays, including antioxidant and anti-inflammatory evaluations.
Figure 3: Chopping and preparation of Kalanchoe pinnata leaves for extraction; the air-dried leaves of Kalanchoe pinnata were cut into small pieces (~1 cm) and then weighed to measure 100 g. The pieces were further chopped until a pulp was obtained, forming the starting material for the extraction process.
Figure 4: Extraction of Kalanchoe pinnata pulp using 99% acetone; the freshly prepared pulp of Kalanchoe pinnata leaves was extracted with 99% acetone in a 2:1 (v/w) ratio. The mixture was left for 72 hours before filtering to obtain the acetone extract.
Figure 5: Filtration and evaporation of Kalanchoe pinnata acetone extract; after 72 hours, the acetone extract was filtered using filter paper to remove plant debris. The filtrate was evaporated to collect a dry powder, used as a concentrated extract for further assays.
Figure 6: Preparation of extract solution for assays; the dry powder collected from Kalanchoe pinnata leaves was dissolved in distilled water to prepare a two-fold dilution series (0.08 g of extract powder in 100 ml of water). The extract solution was refrigerated and used for all antioxidant and anti-inflammatory assays.
A two-fold dilution series was made by dissolving 0.08 g of extract powder in 100 ml of distilled water. It was stored in the refrigerator and used as the sample for all five assays.
Evaluation of in vitro Anti-inflammatory activity of acetone extracts of Kalanchoe pinnata
Egg albumin denaturation activity: The anti-inflammatory activity of unknown crude extracts was determined In vitro for inhibition of denaturation of egg albumin (protein).
0.2 mL of 1% egg albumin solution, 2 mL of sample extract or standard, and 2.8 mL of phosphate-buffered saline (pH 7.4) were mixed to form a reaction mixture of a total volume of 5 mL. The control was made by mixing 2 mL of triple distilled water, 0.2 mL 1% egg albumin solution, and 2.8 mL of phosphate buffered saline to make a total volume of 5 mL. The reaction mixtures were then incubated at 37 ± 2 °C for 30 min and were heated in a water bath at 70 ± 2 °C for 15 min. After cooling, the absorbance was measured at 300 nm by a suitable UV/Vis spectrophotometer using triple distilled water as the blank. The same steps were followed for the standard diclofenac sodium series. The percentage inhibition was calculated compared to a standard using the relationship:
- Percentage inhibition compared to the standard = (Absorbance of test / Absorbance of the standard at [0.8 mg/ml]) × 100% [0.8 mg/ml]) × 100%
HRBC clot lysis activity: The same amount of venous blood was transferred into different pre-weighed sterile microcentrifuge tubes (500 μl/tube). Incubated at 37 °C for 45 minutes. After clot formation, the serum was removed completely. (Aspirated out without disturbing the clot formed using a micropipette). Weigh was taken of each tube having clots again to determine the clot weight. Each micro-centrifuge tube containing the clot was properly labelled. 100 μl of extracts of plants (50, 100, 250, 500, 1000, 2000 μg/ml) with Dimethyl sulfoxide were added to the tubes.
- 100 μl of Aspirin was added to one tube instead of plant extract and this serves as a positive control.
- The same amount of DMSO was added to another tube containing a clot and this serves as a negative thrombolytic control.
All the tubes were incubated at 37 °C for 90 minutes and observed for clot lysis. After incubation, the fluid obtained was removed using a micropipette. The tubes were weighed again to observe the difference in weight after clot disruption. The difference obtained in weight taken before and after clot lysis is expressed as a percentage of clot lysis.
- Percentage of clot lysis compared to standard = [(Test weight before – Test weight after)/ (Standard weight before – standard weight after)] × 100%
Evaluation of in vitro antioxidant activity of acetone/aqueous extracts of five plants
H2O2 free radicals scavenging activity: 0.08 g of plant extract was dissolved in 100 mL and from this 1 ml was taken and a two-fold dilution series was made (10 points). 100 µl of plant extract was taken from each tube from the series and was put into separate labeled tubes. For the standard Ascorbic acid, the same steps were followed. 600 µL of H2O2 was added to the tubes. The volume was made up to 4 mL with phosphate buffer (pH 7.4) in all test tubes. The identical Ascorbic acid series without sample served as the standard (phosphate buffer, H2O2, and Ascorbic acid). All the test tubes were incubated for 10 min at room temperature. The absorbance of the H2O2 solution was measured at 300 nm against the blank. H2O2 scavenging activity was calculated compared to the standard by the Formula.
- Percentage inhibition compared to the standard = (Absorbance of test / Absorbance of the standard at [0.8 mg/ml]) × 100%
DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging anti-oxidant activity assay:
6 x 10-5 M of DPPH in methanol was prepared.
- 7.89 mg for 100 ml,
- for 250 ml = (7.89/100) X 250
- = 19.725 mg
- = 19.725 X 10-3 g
- = 0.019725 g
- = 0.02 g
500 µl of each sample solution was transferred into an Eppendorf tube. Each concentration was tested in triplicate. 500 µl of DPPH solution was transferred and mixed with the sample solution. This was shaken and stood at room temperature for 30 minutes. Absorbance was measured at 520 nm. Ascorbic acid was used as the positive control and distilled water was used as the negative control.
- Percentage inhibition compared to the standard = [(Absorbance of negative control - Absorbance of the test) / Absorbance of the standard at [0.8 mg/ml]] × 100%
FRAP assay: 0.08 g of plant extract was dissolved in 100 ml and from this 1 ml was taken and a two-fold dilution series was made (10 points). In all the test tubes 2.5 ml of PBS was added. The contents in each tube were thoroughly mixed. Then, 2.5 ml of 1% potassium ferricyanide solution was added to all of the samples. After this, each reaction mixture was vortexed well using a vortex shaker. The samples were incubated at 50 °C for around 20 minutes. Once the incubation time was over, 2.5 ml of 10% TCA was added to each sample. The test tubes were centrifuged at 3,000 rpm for 10 minutes. From these centrifuged samples, 2.5 ml supernatant was collected in separate test tubes. After this, in the same new separate test tubes, 0.5 ml of ferric chloride was added. This gave us a bluish color formation. And then the absorbance was measured at 700 nm. A sample having more concentration will show higher absorbance and the opposite is true.
Ascorbic acid was used as the positive control and distilled water was used as the negative control [18].
- Percentage inhibition compared to the standard = (Absorbance of test / Absorbance of the standard at [0.8 mg/ml]) × 100%
The yield of the Extraction of the plant material
The weight of the acetone extract obtained after the extraction of 250 g of Kalanchoe pinnata was 5 g. Therefore, the yield of the hexane extract was 2% w/w. (Table 1).
- Whole plant = (5/250 g) X 100
- Yield = 2%
Graphical illustrations of the comparative study on anti-inflammatory and antioxidant assays of Kalanchoe pinnata
I. Egg albumin denaturation assay
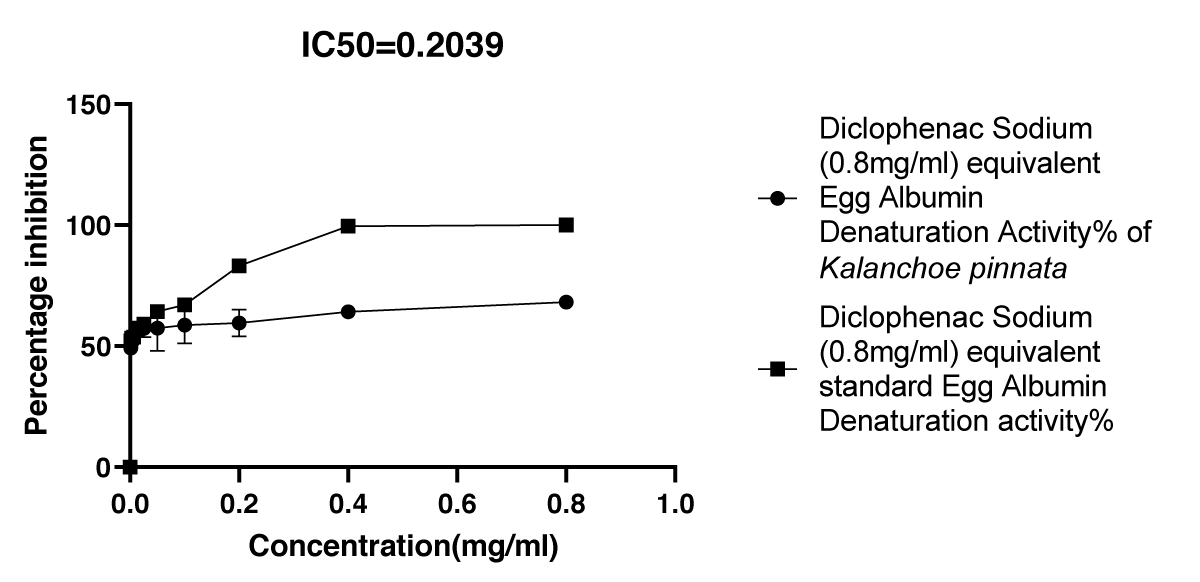
Graphical Illustration 1: Comparative study on anti-inflammatory and antioxidant activities of Kalanchoe pinnata (Acetone extract) and Diclofenac sodium in the Egg Albumin Denaturation Assay.
Graphical Illustration 1
- Observation of egg albumin denaturation assay:
The extract showed a lower percentage inhibition of egg albumin denaturation compared to the standard anti-inflammatory drug, diclofenac sodium, suggesting a less potent anti-inflammatory effect in this assay.
II. HRBC assay
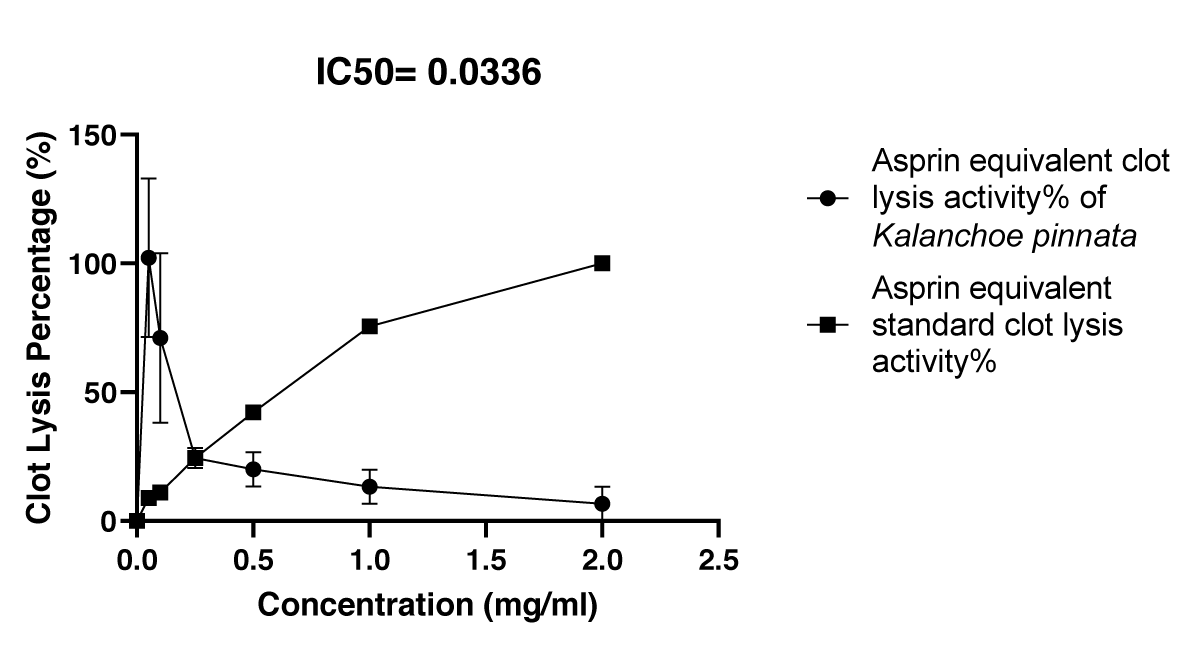
Graphical Illustration 2: Comparative Study on HRBC Clot Lysis Activity of Kalanchoe pinnata Acetone Extract and Aspirin - the HRBC Clot Lysis Assay was conducted to compare the anti-inflammatory effects of Kalanchoe pinnata acetone extract and the standard anti-inflammatory drug, Aspirin.
Graphical Illustration 2
- Observation of HRBC Assay:
Initially, the acetone extract exhibited a higher clot lysis percentage than Aspirin; however, over time, the extract’s clot lysis activity decreased, eventually showing a lower percentage than Aspirin. This suggests a temporal variation in the anti-inflammatory potential of the extract compared to the standard.
III. H2O2 assay
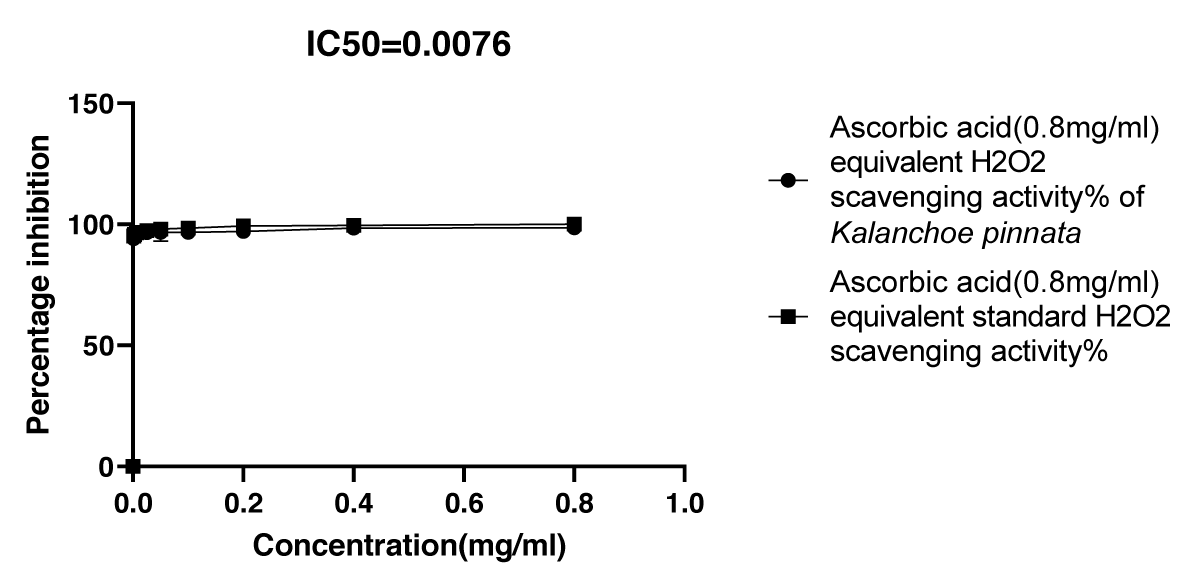
Graphical Illustration 3: Comparative Study on Antioxidant Activity: H2O2 Scavenging Assay of Kalanchoe pinnata Acetone Extract and Ascorbic Acid.
Graphical Illustration 3
- Observation of H2O2 scavenging assay:
The H2O2 Scavenging Assay demonstrated that the acetone extract of Kalanchoe pinnata exhibited a high percentage inhibition of H2O2 (95.8%), comparable to the standard antioxidant, ascorbic acid. This indicates that the acetone extract of Kalanchoe pinnata has a strong antioxidant potential similar to that of ascorbic acid.
IV. DPPH assay
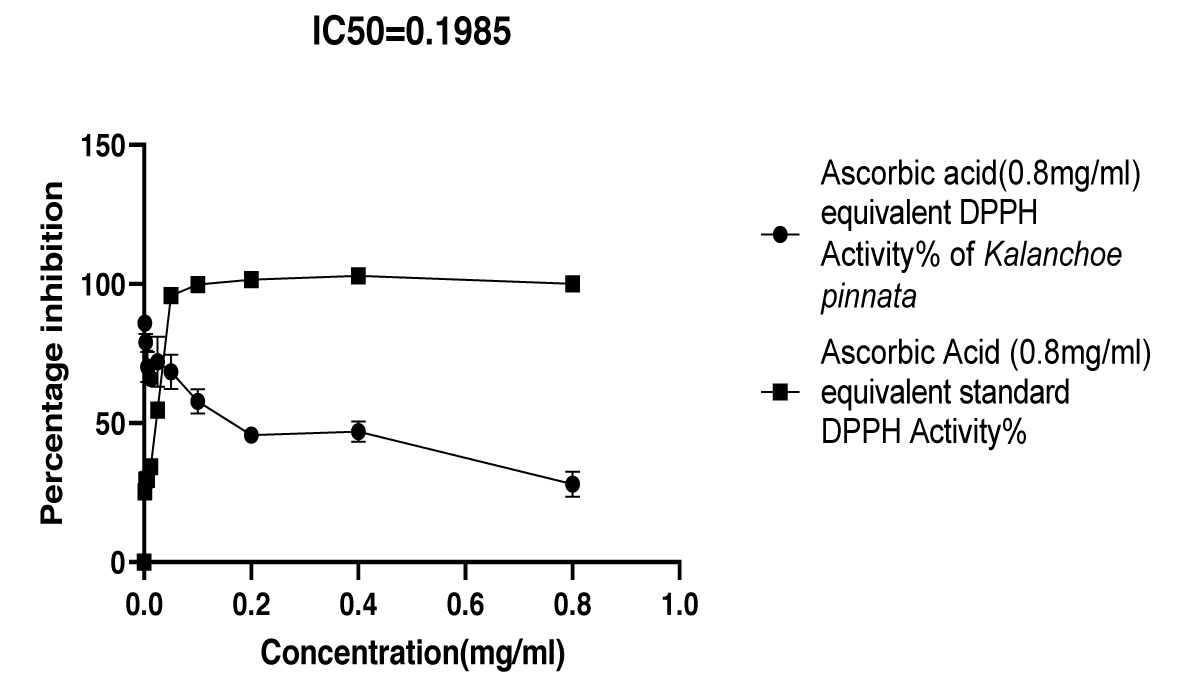
Graphical Illustration 4: Comparative Study on Antioxidant Activity: DPPH Radical Scavenging Assay of Kalanchoe pinnata Acetone Extract and Ascorbic Acid.
Graphical Illustration 4
- Observation of DPPH radical scavenging assay:
The DPPH Radical Scavenging Assay revealed that the acetone extract of Kalanchoe pinnata initially exhibited a higher percentage inhibition of DPPH radicals than the standard antioxidant, ascorbic acid. However, over time, the inhibition percentage of the extract decreased, shifting to a lower level compared to ascorbic acid, indicating a temporal variation in its antioxidant effectiveness.
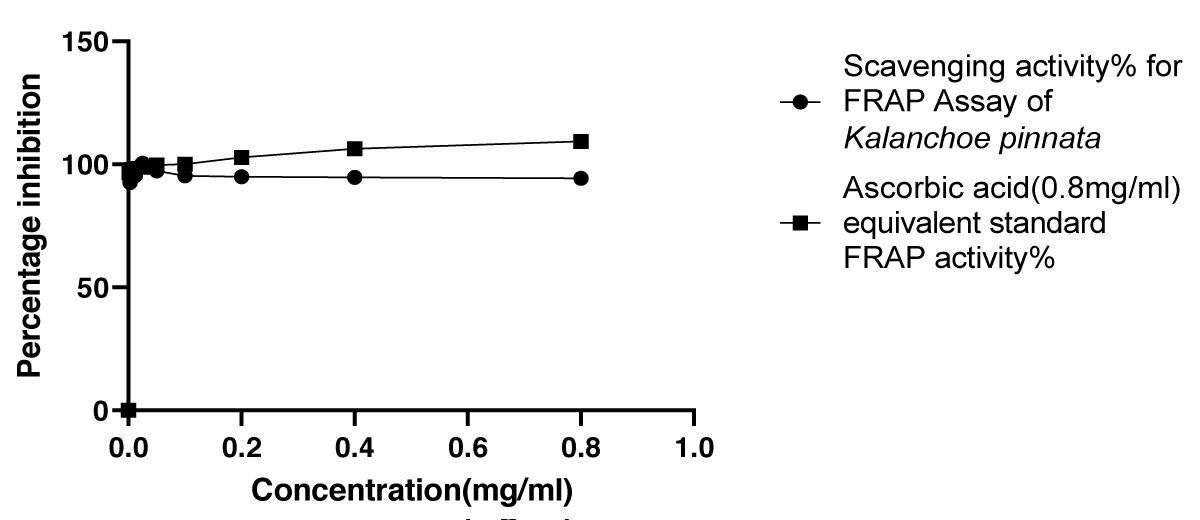
V. FRAP assay
Graphical Illustration 5: Comparative Study on Antioxidant Activity: FRAP Assay of Kalanchoe pinnata Acetone Extract and Ascorbic Acid.
Graphical Illustration 5
- Observation of FRAP assay:
The FRAP Assay demonstrated that the acetone extract of Kalanchoe pinnata exhibited a high antioxidant activity (94.32%), comparable to the standard antioxidant, ascorbic acid. This indicates that the acetone extract of Kalanchoe pinnata possesses strong antioxidant properties, similar to those of ascorbic acid.
Medicinal plants have been extensively studied for their bioactive properties, with various extraction methods employed to maximize the yield of active compounds. Common extraction techniques include Soxhlet extraction, maceration, hydro distillation, ultrasound-assisted extraction, enzyme-assisted extraction, microwave-assisted extraction, and pulsed electric field-assisted extraction [15,17-19]. Each of these methods offers distinct advantages depending on the intended application. In this study, the acetone maceration extraction method was selected due to its simplicity, efficiency, and relatively short processing time. This technique allows for the preservation of key biomolecules in the plant matrix, enabling the effective extraction of active compounds.
The pre-extraction method, specifically grinding the plant material, is crucial for ensuring the homogeneity and optimal yield of active compounds. By carefully selecting these pre-extraction steps, the quality of the extract is preserved, enabling more accurate and reproducible results.
To investigate the bioactivity of Kalanchoe pinnata, particularly its anti-inflammatory and antioxidant properties, several well-established assays were employed. These assays are widely recognized for their reliability and sensitivity in evaluating plant-derived bioactivities [20]. The egg albumin denaturation assay, a widely used model for assessing anti-inflammatory activity, revealed promising results. The acetone extract of Kalanchoe pinnata exhibited significant inhibition of egg albumin denaturation, with an IC50 value of 0.2039 mg/ml, which is comparable to the standard diclofenac sodium (IC50 = 0.2315 mg/ml) [21]. The low discrepancy between the IC50 values suggests that the plant extract possesses anti-inflammatory potential on par with the standard anti-inflammatory agent. Furthermore, the percentage inhibition of albumin denaturation increased proportionally with the concentration of the extract, supporting the conclusion that Kalanchoe pinnata exhibits dose-dependent anti-inflammatory effects.
The HRBC assay, which evaluates the membrane-stabilizing potential of a substance, further validated the anti-inflammatory properties of the extract. At a concentration of 2 mg/ml, the Kalanchoe pinnata acetone extract exhibited significant clot lysis activity, with an IC50 value of 0.0336 mg/ml, demonstrating potent activity compared to the standard aspirin, which had an IC50 value of 1.512 mg/ml [22]. Interestingly, the extract initially showed a higher percentage of clot lysis than aspirin, although this effect decreased over time. This shift in activity could be due to the dynamic nature of the interaction between the bioactive compounds in the extract and the cellular components involved in the clot lysis process. The data suggest that Kalanchoe pinnata has substantial anti-inflammatory effects, supported by its ability to promote membrane stabilization and modulate inflammatory processes effectively.
To further explore the antioxidant potential of the acetone extract, three widely recognized assays—H2O2 free radical scavenging, DPPH radical scavenging, and FRAP—were employed. The H2O2 free radical scavenging assay, which measures the ability to neutralize reactive oxygen species, demonstrated that the acetone extract of Kalanchoe pinnata possesses impressive antioxidant properties, with an IC50 value of 0.0076 mg/ml. This result is comparable to the standard ascorbic acid, which has an IC50 value of 0.0305 mg/ml [23]. The strong H2O2 scavenging activity of the extract further supports the hypothesis that Kalanchoe pinnata contains potent antioxidant compounds capable of mitigating oxidative stress, a key factor in many inflammatory and degenerative diseases.
In the DPPH radical scavenging assay, the acetone extract showed a high degree of radical scavenging activity, particularly at higher concentrations. The extract exhibited a higher percentage inhibition of DPPH radicals at lower concentrations compared to the standard ascorbic acid [24]. While the IC50 value of the extract (0.1985 mg/ml) was slightly higher than that of ascorbic acid (0.02496 mg/ml), the extract demonstrated rapid and effective DPPH scavenging, suggesting a substantial antioxidant capacity.
The FRAP assay, which evaluates the reducing power of a substance, revealed that the acetone extract of Kalanchoe pinnata displayed a high antioxidant potential, with a maximum activity of 94.32% at 0.8 mg/ml. This is nearly equivalent to the antioxidant activity of the standard ascorbic acid (96.66%), further confirming the remarkable reducing power of the plant extract [25]. The high FRAP activity indicates that Kalanchoe pinnata could potentially protect against oxidative damage by donating electrons to neutralize free radicals.
The traditional medicinal uses of Kalanchoe pinnata, including its application in the treatment of burns, boils, insect bites, and various other ailments, are supported by the results of this study [26]. The significant anti-inflammatory and antioxidant activities demonstrated by the acetone extract of Kalanchoe pinnata suggest that the plant may be an effective remedy for conditions associated with inflammation and oxidative stress. Previous studies, such as those showing the reduction of paw edema in mice, align with the findings from this study, further validating the in vivo potential of Kalanchoe pinnata as an anti-inflammatory agent.
In summary, the findings from this study offer compelling evidence for the significant anti-inflammatory and antioxidant potential of Kalanchoe pinnata. The acetone extract demonstrated potent activity in various bioassays, comparable to that of established standards like diclofenac sodium, aspirin, and ascorbic acid. The plant’s ability to effectively neutralize reactive oxygen species and inhibit inflammation, as shown by the H2O2 scavenging, DPPH radical scavenging, and FRAP assays, suggests that it could be a valuable resource for managing oxidative stress and inflammatory disorders.
These results not only validate the traditional medicinal uses of Kalanchoe pinnata but also underscore its therapeutic potential in modern medicine. The relatively low discrepancy in activity between the plant extract and standard compounds further supports the idea that Kalanchoe pinnata could offer an accessible and effective alternative for inflammation and oxidative stress management. Based on these promising results, further research to isolate and identify the specific bioactive compounds responsible for these effects is recommended.
Thus, this study paves the way for future investigations into the full medicinal potential of Kalanchoe pinnata, enhancing its appeal as a natural and effective therapeutic agent in both clinical and pharmaceutical applications [27-30].
In conclusion, the results of this study clearly demonstrate that Kalanchoe pinnata possesses significant anti-inflammatory and antioxidant properties, with the acetone extract showing comparable efficacy to well-established reference compounds such as diclofenac sodium, aspirin, and ascorbic acid. Through a series of well-validated assays, including egg albumin denaturation, HRBC clot lysis, H2O2 scavenging, DPPH radical scavenging, and FRAP activity, this study provides comprehensive evidence of the plant’s potential as a natural remedy for conditions related to inflammation and oxidative stress.
The acetone extract of Kalanchoe pinnata exhibited dose-dependent anti-inflammatory activity, as evidenced by its significant inhibition of egg albumin denaturation and clot lysis, demonstrating its potential as an anti-inflammatory agent. Furthermore, the plant’s ability to scavenge reactive oxygen species and neutralize free radicals was shown to be comparable to that of the standard antioxidants, ascorbic acid, and diclofenac sodium, suggesting its therapeutic potential in managing oxidative stress-related diseases.
These findings strongly support the ethnobotanical use of Kalanchoe pinnata in traditional medicine and highlight its potential for development into a therapeutic agent for treating a range of inflammatory and oxidative stress-related conditions. Future studies are recommended to isolate and identify the specific bioactive compounds responsible for these activities, as this will help optimize the plant’s therapeutic potential. Additionally, conducting in vivo studies and clinical trials would provide valuable insights into the practical applications and safety of Kalanchoe pinnata as a natural anti-inflammatory and antioxidant agent.
This research lays the foundation for further exploration of Kalanchoe pinnata as a promising candidate for the development of novel, plant-based therapeutics, thereby contributing to the growing interest in natural products as alternatives to conventional pharmaceutical treatments.
I would like to sincerely acknowledge the Department of Biomedical Science, Faculty of Health Sciences, KAATSU International University, Sri Lanka, for providing the facilities and resources necessary to conduct this research. I extend my heartfelt gratitude to Mrs. Nirmani Samarakoon, Senior Lecturer, Department of Biomedical Science, for her invaluable guidance and support throughout the study. I also wish to express my deep appreciation to Professor Malitha Aravinda Siriwardhene, Department of Pharmacy and Pharmaceutical Sciences, Faculty of Allied Health Sciences, University of Sri Jayewardenepura, for his collaboration and supervision, which greatly contributed to the success of this research.
- Kumar Biswas S, Chowdhury A, Das J, Hosen SM, Uddin R, Rahaman MS. A review of the traditional medicinal uses of Kalanchoe pinnata (Crassulaceae). Int J Pharm Pharmacol. 2021;10(1):1–5. Available from: https://www.internationalscholarsjournals.com/articles/a-review-of-the-traditional-medicinal-uses-of-kalanchoe-pinnata-crassulaceae.pdf
- Zhang X, Sun Y, Chen L, Li Y, Yang L. Pharmacological properties and potential medicinal applications of Kalanchoe pinnata: A comprehensive review. Phytochem Rev. 2018;17(2):305–318.
- Anandan J, Shanmugam R. Antioxidant, anti-inflammatory, and antimicrobial activity of the Kalanchoe pinnata and Piper longum formulation against oral pathogens. Cureus. 2024;16(4). Available from: https://doi.org/10.7759/cureus.57824
- Quintero EJ, León EG de, Morán-Pinzón J, Mero A, León E, Cano LPP, Rica C. Evaluation of the leaf extracts of Kalanchoe pinnata and Kalanchoe daigremontiana chemistry, antioxidant and anti-inflammatory activity. EJMP. 2021;32(5):45–54. Available from: https://doi.org/10.9734/EJMP/2021/v32i530392
- Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49(11):1603–1616. Available from: https://doi.org/10.1016/j.freeradbiomed.2010.09.006
- Verma S. Medicinal plants with anti-inflammatory activity. J Phytopharmacol. 2016;5(4):157–159. Available from: https://doi.org/10.31254/phyto.2016.5407
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4(8):118–126. Available from: https://doi.org/10.4103/0973-7847.70902
- Ferreira RT, Araújo M, Coutinho S, Malvar C, Costa EA, Florentino IF, Vanderlinde FA. Mechanisms underlying the antinociceptive, antiedematogenic, and anti-inflammatory activity of the main flavonoid from Kalanchoe pinnata. 2014. Available from: https://doi.org/10.1155/2014/429256
- Saeed K, Farhan M, Chughtai J, Khaliq A, Mehmood T, Khalid MZ, Lengemukonzo E. Impact of extraction techniques and process optimization on antioxidant and antibacterial potential of Kalanchoe pinnata leaf extract. Int J Food Prop. 2024;27(1):909–926. Available from: https://doi.org/10.1080/10942912.2024.2373796
- Calder PC. Eicosanoids. Essays Biochem. 2020;64(3):423–441. Available from: https://doi.org/10.1042/EBC20190083
- Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: Structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69(1):145–182. Available from: https://doi.org/10.1146/annurev.biochem.69.1.145
- Adinortey MB, Galyuon IKA, Asamoah NO. Kalanchoe pinnata: A review of its ethnomedicinal uses, biological activities, and phytochemical constituents. J Med Plants Res. 2013;7(14):2338–2346.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28(1):25–30. Available from: https://doi.org/10.1016/S0023-6438(95)80008-5
- Griess P. On a new reagent for nitrous acid. Ann Chem. 1879;130(2):307–310.
- Zawirska-Wojtasiak R, Jankowska B, Piechowska P, Mildner-S. Vitamin C and aroma composition of fresh leaves from Kalanchoe pinnata and Kalanchoe daigremontiana. Sci Rep. 2019;1–8. Available from: https://doi.org/10.1038/s41598-019-56359-1
- Benjamaa R, Elbouny H, Errati H, Moujanni A, Kaushik N, Gupta R. Comparative evaluation of antioxidant activity, total phenolic content, anti-inflammatory, and antibacterial potential of Euphorbia-derived functional products. Front Pharmacol. 2024;1–18. Available from: https://doi.org/10.3389/fphar.2024.1345340
- Rajsekhar PB, Arvind Bharani RS, Ramachandran M, Jini Angel K, Sharadha Priya Vardhini. The “Wonder Plant” Kalanchoe pinnata (Linn.) Pers.: A review. J Appl Pharm Sci. 2016;6(3):151–158. Available from: https://doi.org/10.7324/JAPS.2016.60326
- Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. Springer Science & Business Media; 1998. Available from: https://doi.org/10.1007/978-94-009-5921-7
- Mizushima Y, Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol. 1968;20(3):169–173. Available from: https://doi.org/10.1111/j.2042-7158.1968.tb09718.x
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144–158. Available from: https://doi.org/10.5344/ajev.1965.16.3.144
- Oyaizu M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet. 1986;44(6):307–315. Available from: https://doi.org/10.5264/eiyogakuzashi.44.307
- Sharma N. Antioxidant and anti-inflammatory properties of medicinal plants: A review. Asian J Pharm Clin Res. 2016;9(4):33–36.
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269(2):337–341. Available from: https://doi.org/10.1006/abio.1999.4019
- Mizushima Y, Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol. 1968;20(3):169–173. Available from: https://doi.org/10.1111/j.2042-7158.1968.tb09718.x
- Timsina B. Evaluation of antioxidant, anti-inflammatory, and antimicrobial activity of medicinal plants. J Med Plants Res. 2017;11(22):362–370.
- Akinmoladun FO. Phytochemical constituents and antioxidant properties of extracts from the leaves of Chromolaena odorata. Sci Res Essays. 2010;5(16):2201–2205.
- Ramon P, Bergmann D, Abdulla H, Sparks J, Omoruyi F. Bioactive ingredients in K. pinnata extract and synergistic effects of combined K. pinnata and metformin preparations on antioxidant activities in diabetic and non-diabetic skeletal muscle cells. Int J Mol Sci. 2023;24(7):6211. Available from: https://doi.org/10.3390/ijms24076211
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect. 2001;109(Suppl 1):69–75. Available from: https://doi.org/10.1289/ehp.01109s169
- Kamboj A, Saluja AK. Bryophyllum pinnatum (Lam.) Kurz: Phytochemical and pharmacological profile: A review. Pharmacogn Rev. 2009;3(6):364–374. Available from: https://phcogrev.com/article/2009/3/6-13
- Ojewole JA. Antinociceptive, anti-inflammatory and antidiabetic properties of Bryophyllum pinnatum (Crassulaceae) leaf aqueous extract. J Ethnopharmacol. 2005;99(1):13–19. Available from: https://doi.org/10.1016/j.jep.2005.01.025